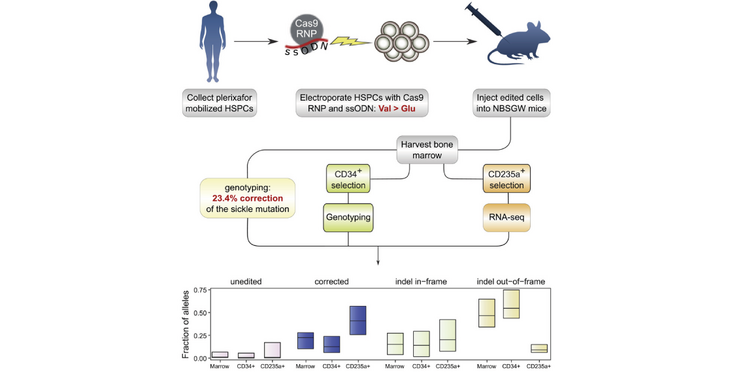
A publication with co-corresponding author Jacob Corn reports that mutated B-globin can be corrected with CRISPR in patient derived cells ex vivo and then injected into mice for analysis. This analysis revealed that over 30% of the hematopoietic stem cells that engrafted over a longer term carried at least one corrected allele. The use of a high-fidelity Cas9 variant reduced the off targets to a minimum. Interstingly, cells harboring corrected alleles got enriched along the differentiation pathway, indicating that these erytrhoblasts have a fitness advantage. The findings were published in the article "High-level correction of the sickle mutation is amplified in vivo during erythroid differentiation" in the journal iScience.
Highlights
- The gene editing protocol corrects the sickle mutation in ~30% of engrafting cells
- Random assortment of engrafting stem cell clones without clonal dominance was shown
- Corrected erythroid cells are preferentially enriched compared with unedited cells
Summary
Background:
A point mutation in sickle cell disease (SCD) alters one amino acid in the B-globin subunit of hemoglobin, with resultant anemia and multiorgan damage that typically shortens lifespan by decades. Because SCD is caused by a single mutation, and hematopoietic stem cells (HSCs) can be harvested, manipulated, and returned to an individual, it is an attractive target for gene correction.
Results:
An optimized Cas9 ribonucleoprotein (RNP) with an ssDNA oligonucleotide donor together generated correction of at least one B-globin allele in more than 30% of long-term engrafting human HSCs. After adopting a high-fidelity Cas9 variant, efficient correction with minimal off-target events also was observed. In vivo erythroid differentiation markedly enriches for corrected ?-globin alleles, indicating that erythroblasts carrying one or more corrected alleles have a survival advantage.
Significance
These findings indicate that the sickle mutation can be corrected in autologous HSCs with an optimized protocol suitable for clinical translation.
Read the Publication in iScience (Open Access)
Figure, highlights, summary and title from Magis, de Witt et al. (2022) iScience published under a CC BY-NC-ND 4.0 license.