The Allain, Sharma, Jonas and Leitner groups studied the interaction between the ubiquitin-like domain of SF3A1, which is part of the U2 snRNP, and stemloop 4 of the U1snRNP applying a variety of methods. The stemloop 4 of U1 snRNP is sequence specifically recognized by an intrinsically disordered RGG motif and this interaction is involved in early spliceosome assembly. Their findings have been published in the article "Sequence-specific RNA recognition by an RGG motif connects U1 and U2 snRNP for spliceosome assembly" in PNAS.
Significance
Pre-messenger RNA (pre-mRNA) splicing is a key regulatory step in gene expression. The splicing reaction is mediated by the spliceosome, a dynamic complex comprising five small nuclear ribonucleoproteins (snRNPs), which assembles onto each intron in multiple steps. We present detailed structural analysis and supporting functional data of an important protein–RNA interaction between human U1 and U2 snRNP. Our structure shows that an intrinsically disordered arginine-glycine (RGG/RG)–rich motif of a U2 snRNP subunit forms an RNA-sequence–specific connection with U1 snRNP. This study broadens the functional scope of unstructured RGG/RG-rich motifs in RNA binding proteins and provides a molecular basis of early steps of spliceosome assembly, which may help develop innovative therapeutic strategies against diseases originating from splicing defects.
Abstract
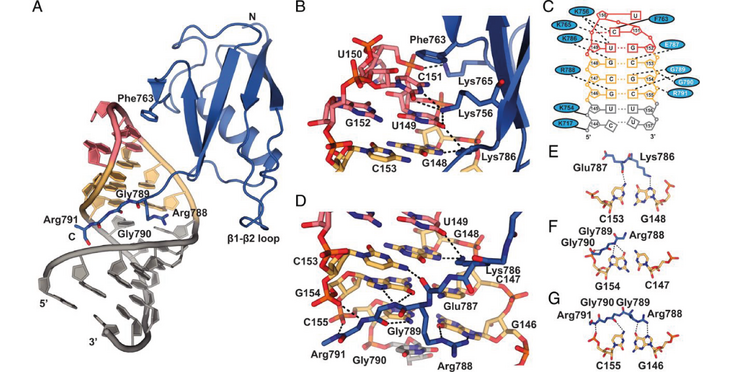
In mammals, the structural basis for the interaction between U1 and U2 small nuclear ribonucleoproteins (snRNPs) during the early steps of splicing is still elusive. The binding of the ubiquitin-like (UBL) domain of SF3A1 to the stem-loop 4 of U1 snRNP (U1-SL4) contributes to this interaction. Here, we determined the 3D structure of the complex between the UBL of SF3A1 and U1-SL4 RNA. Our crystallography, NMR spectroscopy, and cross-linking mass spectrometry data show that SF3A1-UBL recognizes, sequence specifically, the GCG/CGC RNA stem and the apical UUCG tetraloop of U1-SL4. In vitro and in vivo mutational analyses support the observed intermolecular contacts and demonstrate that the carboxyl-terminal arginine-glycine-glycine-arginine (RGGR) motif of SF3A1-UBL binds sequence specifically by inserting into the RNA major groove. Thus, the characterization of the SF3A1-UBL/U1-SL4 complex expands the repertoire of RNA binding domains and reveals the capacity of RGG/RG motifs to bind RNA in a sequence-specific manner.
Read the Publication in PNAS (Open Access)
Abstract, figure, significance and title from de Vries, Martelly, Campagne et al. (2022) PNAS published under a CC BY-NC-ND 4.0 license.