How does metabolism intersect with fundamental principles in translation regulation? And how does introducing a pro-differentiation proteome lead to cures in highly malignant mouse models via a "Reverse Yamanaka-Effect"? Read more about this recent NCCR RNA & Disease publication!
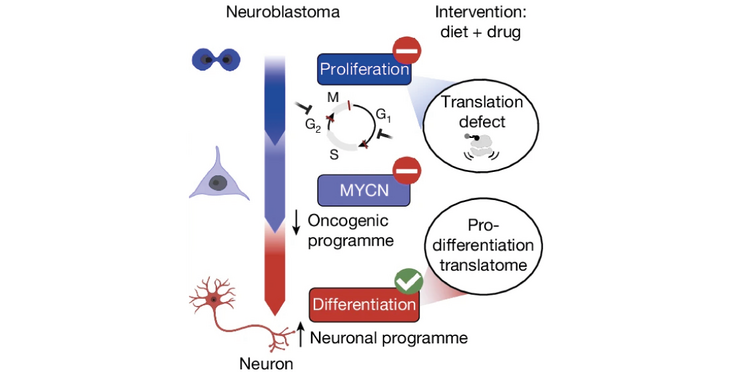
Paediatric cancers display distinct hallmarks as compared with those affecting adults and therefore harbour specific vulnerabilities to treatment. One such hallmark is arrested cellular differentiation, as seen in childhood neuroblastoma. The Morscher lab and collaborators, including the Leidel group, showed that a combination of an approved drug treatment with an arginine- and proline-deficient diet profoundly affects survival in a highly lethal mouse model of the tumour and xenograft models with patient-derived cells. In addition to the therapeutic implications to be tested in the clinic, their mechanistic findings revealed alterations in translation and point to the existence of codon-based translational rewiring upon metabolic changes.
Neuroblastoma is a highly lethal childhood cancer, mainly affecting kids below five years of age. Across the board, neuroblastomas show increased polyamine synthesis due to overexpression of the rate-limiting enzyme ornithine decarboxylase, and its inhibition by difluoromethylornithine (DFMO) has recently been approved by the FDA. The substrate of polyamine biosynthesis, ornithine, is ultimately derived from arginine or via an alternative synthesis from proline and glutamine. Importantly, by introducing metabolic labelling studies using stable isotopes, the researchers found that neuroblastomas feed on circulating ornithine.
As circulating ornithine is produced in healthy organs from arginine and proline, the team then tested the effect of a proline- and arginine-free diet combined with the polyamine synthesis inhibitor DFMO. Most strikingly, despite using a highly lethal mouse model, this simple metabolic intervention induced tumour-free survival in a third of the mice for over a year. Not a single mouse was cured with the FDA-approved drug DFMO alone, while one-fifth of the mice receiving the DFMO and a proline- and arginine-free diet combination remained long-term tumour-free.
The researchers then focused on extensive mechanistic experiments to shed light on the translation biology underlying the observed anti-tumour effects. They found that the diet enhanced polyamine depletion by more than two-fold beyond DFMO treatment. As polyamines are known to promote translation, and the amino acids proline and arginine are building blocks of proteins, the researchers then applied ribosome profiling to examine the effects of the therapy on translation in more detail.
To their great surprise, they found that the effects of the combined treatment intervention were completely independent of amino acid abundance (tRNA charging) or previously known effects of polyamine depletion. Classically, the polyamine spermidine is highlighted in the context of modifying the translation factor eIF5A. Whereas eIF5A hypusination was confirmed to be reduced, this was not relevantly driving the phenotype. When zooming in on the ribosome profiling data, the researchers found an unexpected translational issue.
Ribosomes were predominantly stalled at codons harbouring adenosine (A) at their third position, while codons containing a guanosine in the third position were less occupied under the combination treatment. The tRNAs required for decoding these stalled codons are highly modified at the nucleotide that pairs with the third codon nucleotide. So, polyamine depletion seems to cause codon-specific translation defects independent of eIF5A. "Discovering this eIF5a independent mechanism affecting translation in response to polyamine depletion was a big surprise and a paradigm shift for the field," says Raphael Morscher, group leader at the University Children's Hospital Zurich and last author of the study published in Nature.
When adding the transcriptomic and proteomic layers to their analysis, the authors found that expression of proteins changed in relation to their fraction of codons with an A in the third position. This specifically depleted cell cycle-related proteins, which showed disturbed translation because of their enrichment of such codons with an A at the third position. In contrast, translation of proteins associated with neuronal differentiation, which contain fewer A-ending codons, was increased. Reduced cell cycle proteins and increased differentiation proteins reprogrammed neuroblastoma cells towards differentiation, a process arrested in this cancer. "I like to call this induction of differentiation by inducing a pro-differentiation proteome the Reverse-Yamanaka effect," comments Morscher.
In conclusion, the authors raise the possibility that codon usage and metabolism have co-evolved to change the proteome in a coordinated fashion in response to metabolomic changes. In this case, polyamine depletion via codons with an A in the third position leads to a proteome change that disfavours division and promotes differentiation. As to the natural benefit, the researchers think the purpose is to steer developmental decisions. But now they want to assess whether this system can be harnessed to improve the treatment of childhood neuroblastoma in the clinic.
Read the Publication in Nature (Open Access)
Figure from Cherkaoui et al. (2025) Nature published under a CC BY 4.0 license.