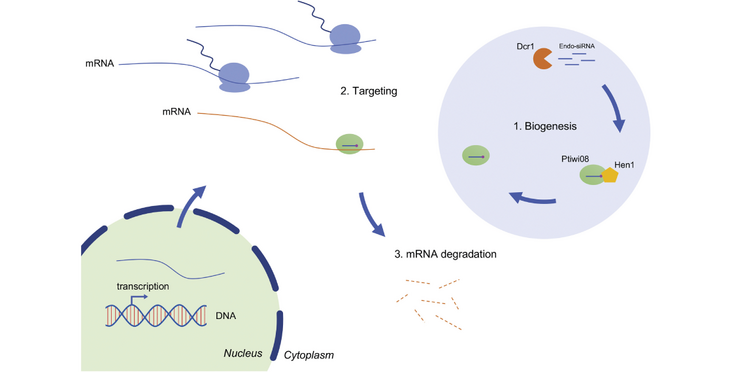
The ciliate Paramecium tetraurelia's genome enodes many PIWI-clade proteins, of many of which the function is unkown. One of them is called Ptiwi08, which is expressed only shortly during development. The Nowacki lab found out that Ptiwi08 degrades untranslated mRNAs at this developmental transition point. Their findings have been published in the article "Developmental mRNA clearance by PIWI-bound endo-siRNAs in Paramecium" in Cell Reports.
Highlights
- Discovery of a small-RNA-mediated mRNA clearance pathway in a unicellular eukaryote
- Endo-siRNAs display a strict strand bias and are antisense to their target mRNAs
- Endo-siRNAs are Dicer dependent and bound by a PIWI-clade Argonaute protein, Ptiwi08
- Untranslated mRNAs are likely targeted for degradation by the Ptiwi08 pathway
Abstract
The clearance of untranslated mRNAs by Argonaute proteins is essential for embryonic development in metazoans. However, it is currently unknown whether similar processes exist in unicellular eukaryotes. The ciliate Paramecium tetraurelia harbors a vast array of PIWI-clade Argonautes involved in various small RNA (sRNA) pathways, many of which have not yet been investigated. Here, we investigate the function of a PIWI protein, Ptiwi08, whose expression is limited to a narrow time window during development, concomitant with the start of zygotic transcription. We show that Ptiwi08 acts in an endogenous small interfering RNA (endo-siRNA) pathway involved in the clearance of untranslated mRNAs. These endo-siRNAs are found in clusters that are strictly antisense to their target mRNAs and are a subset of siRNA-producing clusters (SRCs). Furthermore, the endo-siRNAs are 2'-O-methylated by Hen1 and require Dcr1 for their biogenesis. Our findings suggest that sRNA-mediated developmental mRNA clearance extends beyond metazoans and may be a more widespread mechanism than previously anticipated.
Read the Publication in Cell Reports (Open Access)
Abstract, figure, highlights and title from Solberg et al. (2023) Cell Reports published under a CC BY-NC-ND 4.0 license.