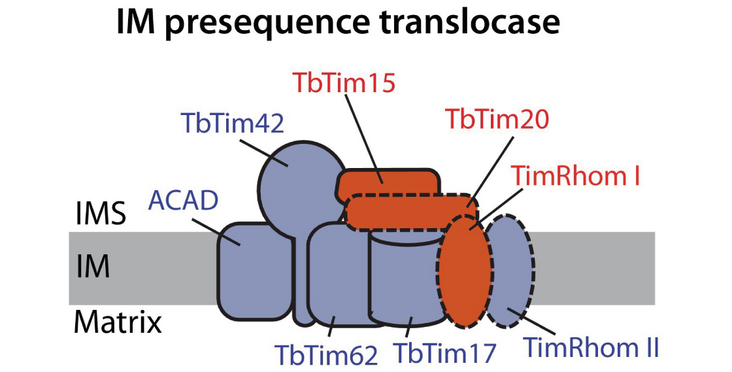
Unlike other organisms, trypanosomes have only one translocases of the inner membrane (TIM) complex to import proteins across the inner mitochondrial membrane. The Schneider lab and collaborators now investigated one of its subunits, TbTim20, which is unlike others not essential. They showed that TbTim20 becomes more important in cells that are harboring a specific mutation in an ATPase subunit, lacking a mitochondrial genome and possessing a low membrane potential. In conclusion, they propose that the protein ins involved in the mitochondrial import of proteins whose import is hypersensitive to low membrane potential. Their findings have been published in the article “TbTim20 facilitates protein import at a low membrane potential in trypanosomes lacking the mitochondrial genome” in the FEBS Journal.
Abstract
Protein import across the mitochondrial inner membrane typically depends on two protein translocases of the inner membrane (TIM) complexes and the membrane potential. The protozoan parasite Trypanosoma brucei, however, has a single, divergent TIM complex. Unlike other trypanosomal TIM subunits, TbTim20 is not essential for the normal growth of the insect or bloodstream forms of T. brucei, leaving its role uncertain. Specific mutations in the y-subunit of the F1FO-ATPase, such as yL262P, permit bloodstream form trypanosomes to grow without mitochondrial DNA (kinetoplast or kDNA). Here, we show that RNAi-mediated depletion of TbTim20 inhibits growth of this cell line, but only if it lacks the kDNA. Titration of mitochondrial uncouplers and direct membrane potential measurements reveal that TbTim20 becomes more critical as the membrane potential decreases across all tested cell lines. Proteomic analysis of the uninduced and induced yL262P TbTim20-RNAi cell line, which lacks kDNA and exhibits the lowest membrane potential, shows depletion of a subset of imported proteins. This subset includes ATPase subunits, suggesting a mechanism by which TbTim20-silenced cell lines become more sensitive to uncouplers. Thus, we propose that TbTim20 supports the import of a subset of proteins whose import is hypersensitive to a low membrane potential.
Read the Publication in the FEBS Journal (Open Access)
Abstract, figure and title from von Känel C., Aeschlimann S. et al. (2025) FEBS J published under a CC BY 4.0 license.