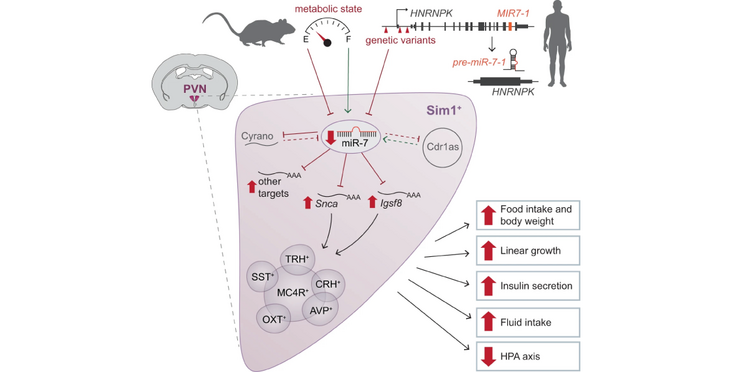
The Stoffel lab (ETH Zurich) in collaboration with the Farooqi lab (University of Cambridge) identified microRNA-7 as a regulator of metabolism, whose deletion leads to obesity in mice. As miRNA-7 is the most highly expressed miRNA in the hypothalamus, the researchers tested several hypothalamus cell types known to be involved in control of metabolism, and found that deletion of miRNA-7 in Sim 1 neurons alone also led to obesity, hyperphagia (overeating) and increased growth. In humans, expression reducing mutations in the genetic locus containing miRNA-7-1 are associated with increased height and body fat. Their findings haven been published in the Nature Communications article "MicroRNA-7 regulates melanocortin circuits involved in mammalian energy homeostasis".
Abstract
MicroRNAs (miRNAs) modulate physiological responses by repressing the expression of gene networks. We found that global deletion of microRNA-7 (miR-7), the most enriched miRNA in the hypothalamus, causes obesity in mice. Targeted deletion of miR-7 in Single-minded homolog 1 (Sim1) neurons, a critical component of the hypothalamic melanocortin pathway, causes hyperphagia, obesity and increased linear growth, mirroring Sim1 and Melanocortin-4 receptor (MC4R) haplo-insufficiency in mice and humans. We identified Snca (alpha-Synuclein) and Igsf8 (Immunoglobulin Superfamily Member 8) as miR-7 target genes that act in Sim1 neurons to regulate body weight and endocrine axes. In humans, MIR-7-1 is located in the last intron of HNRNPK, whose promoter drives the expression of both genes. Genetic variants at the HNRNPK locus that reduce its expression are associated with increased height and truncal fat mass. These findings demonstrate that miR-7 suppresses gene networks involved in the hypothalamic melanocortin pathway to regulate mammalian energy homeostasis.
Read the Publication in Nature Communications (Open Access)
Abstract, figure and title from LaPierre et al. (2022) Nature Commun published under a CC BY 4.0 license.