The Schneider, Warscheid and Vögtle lab characterized the linker protein p166 of the Tripartite Attachment Complex (TAC) in Trypanosomes. Trypanosomes have only one mitochondrium with a single genome called kinetoplast DNA (kDNA). The TAC, which links the kDNA to the base of the flagellum over the two mitochondrial membranes is required for correct kDNA segregation. Their findings have been published in the article "p166 links membrane and intramitochondrial modules of the trypanosomal tripartite attachment complex" in PLoS Pathogens.
Author Summary
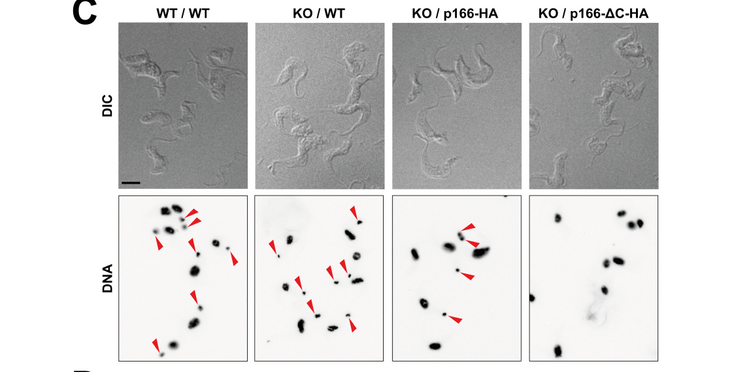
Mitochondria evolved from a single endosymbiotic event and are a hallmark of eukaryotes. The large majority of genes for mitochondrial proteins are nuclear encoded now and only a small number are found in the mitochondrial genome. The protist Trypanosoma brucei is an extreme eukaryote in many aspects. For instance, trypanosomes have a single mitochondrion and its genome–called kinetoplast DNA (kDNA)–locates as a single unit inside the mitochondrion close to the basal body of the flagellum. The tripartite attachment complex (TAC) forms a connection between the basal body and the kDNA ensuring faithful segregation of kDNA among the daughter cells upon cytokinesis. Recently, several TAC subunits of the cytoplasm, the outer mitochondrial membrane (OM) and the mitochondrial matrix have been characterized. Here, we identify p166 as the first TAC subunit of the inner mitochondrial membrane. It is anchored with a single transmembrane domain separating the protein into a N-terminal moiety located in the matrix and a short C-tail. The latter reaches into the intermembrane space and binds the OM subunit TAC60 whereas the N-terminus interacts with the matrix subunit TAC102. Thus, with p166 we identified the missing link required to connect different modules of the TAC.
Abstract
The protist parasite Trypanosoma brucei has a single mitochondrion with a single unit genome termed kinetoplast DNA (kDNA). Faithfull segregation of replicated kDNA is ensured by a complicated structure termed tripartite attachment complex (TAC). The TAC physically links the basal body of the flagellum with the kDNA spanning the two mitochondrial membranes. Here, we characterized p166 as the only known TAC subunit that is anchored in the inner membrane. Its C-terminal transmembrane domain separates the protein into a large N-terminal region that interacts with the kDNA-localized TAC102 and a 34 aa C-tail that binds to the intermembrane space-exposed loop of the integral outer membrane protein TAC60. Whereas the outer membrane region requires four essential subunits for proper TAC function, the inner membrane integral p166, via its interaction with TAC60 and TAC102, would theoretically suffice to bridge the distance between the OM and the kDNA. Surprisingly, non-functional p166 lacking the C-terminal 34 aa still localizes to the TAC region. This suggests the existence of additional TAC-associated proteins which loosely bind to non-functional p166 lacking the C-terminal 34 aa and keep it at the TAC. However, binding of full length p166 to these TAC-associated proteins alone would not be sufficient to withstand the mechanical load imposed by the segregating basal bodies.
Read the Publication in PLoS Pathogens (Open Access)
Abstract, author summary, figure and title from Schimanski et al. PLoS Pathogens (2022) published under a CC BY 4.0 license.