The Zeb1/miR200 axis has been implicated in the regulation of a crucial transition in cancer. However, in vivo studies of the relevance of this axis have been lacking. The lab of NCCR RNA & Disease principal investigator Markus Stoffel now reports their in vivo findings regarding this axis in a Nature Communications article entitled "Genetic dissection of the miR-200-Zeb1 axis reveals its importance in tumor differentiation and invasion".
Abstract:
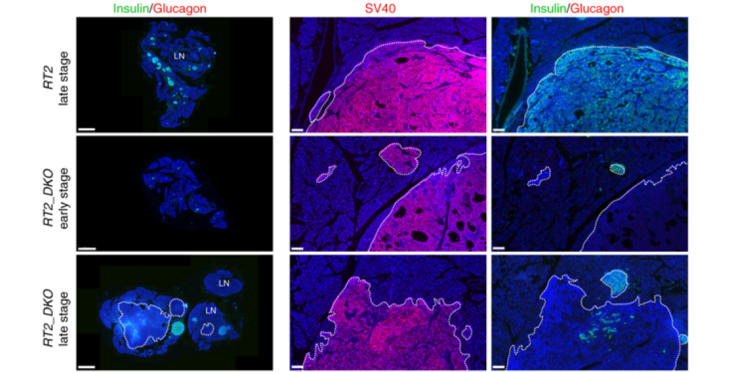
The epithelial-to-mesenchymal transition (EMT) is an important mechanism for cancer progression and metastasis. Numerous in vitro and tumor-profiling studies point to the miR-200–Zeb1 axis as crucial in regulating this process, yet in vivo studies involving its regulation within a physiological context are lacking. Here, we show that miR-200 ablation in the Rip-Tag2 insulinoma mouse model induces beta-cell dedifferentiation, initiates an EMT expression program, and promotes tumor invasion. Strikingly, disrupting the miR-200 sites of the endogenous Zeb1 locus causes a similar phenotype. Reexpressing members of the miR-200 superfamily in vitro reveals that the miR-200c family and not the co-expressed and closely related miR-141 family is responsible for regulation of Zeb1 and EMT. Our results thus show that disrupting the in vivo regulation of Zeb1 by miR-200c is sufficient to drive EMT, thus highlighting the importance of this axis in tumor progression and invasion and its potential as a therapeutic target.
Read the Publication in Nature Communications (Open Access)
Abstract and figure image from Title et al. (2018) Nature Communications, 9(1),4671 published under the CC BY 4.0 license.