The Polymenidou lab in collaboration with the Stahlberg and Lashley groups investigated the seeding and fibril properties of two different pathological FTLD-TDP aggregates. Their findings have been published in EMBO Reports in an article entitled "FTLD-TDP assemblies seed neoaggregates with subtype-specific features via a prion-like cascade".
Synopsis
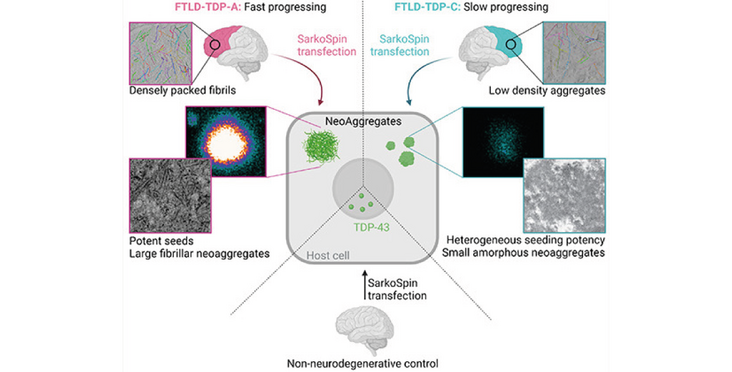
Pathological TDP-43 derived from FTLD patient brains triggers de novo aggregation of physiological TDP-43 in host cells via a prion-like cascade. Different subtypes of FTLD-TDP show distinct organization of TDP-43 aggregates in patient brains, different seeding potencies, neoaggregate structures and C-terminal serine phosphorylation timelines.
- Pathological TDP-43 triggers template-dependent aggregation and amplification of neoaggregates
- FTLD-TDP-A and FTLD-TDP-C aggregates have different seeding potency (high vs. low) and follow distinct timelines of C-terminal serine phosphorylation
- Neoaggregates triggered by FTLD-TDP-A and FTLD-TDP-C display different aggregation profiles (fibrillar vs. amorphous) resembling the original aggregates in patient brains (densely vs. loosely packed fibrils)
Abstract
Morphologically distinct TDP-43 aggregates occur in clinically different FTLD-TDP subtypes, yet the mechanism of their emergence and contribution to clinical heterogeneity are poorly understood. Several lines of evidence suggest that pathological TDP-43 follows a prion-like cascade, but the molecular determinants of this process remain unknown. We use advanced microscopy techniques to compare the seeding properties of pathological FTLD-TDP-A and FTLD-TDP-C aggregates. Upon inoculation of patient-derived aggregates in cells, FTLD-TDP-A seeds amplify in a template-dependent fashion, triggering neoaggregation more efficiently than those extracted from FTLD-TDP-C patients, correlating with the respective disease progression rates. Neoaggregates are sequentially phosphorylated with N-to-C directionality and with subtype-specific timelines. The resulting FTLD-TDP-A neoaggregates are large and contain densely packed fibrils, reminiscent of the pure compacted fibrils present within cytoplasmic inclusions in postmortem brains. In contrast, FTLD-TDP-C dystrophic neurites show less dense fibrils mixed with cellular components, and their respective neoaggregates are small, amorphous protein accumulations. These cellular seeding models replicate aspects of the patient pathological diversity and will be a useful tool in the quest for subtype-specific therapeutics.
Read the Publication in EMBO Reports (Open Access)
Abstract, figure, synopsis and title from De Rossi, Lewis, et al. (2021) EMBO Reports published under a CC BY-NC-ND 4.0 license.