A recent "Molecular Cell" paper by the Corn group in collaboration with the Jonas and Zavolan groups, all three member labs of the NCCR RNA & Disease, demonstrates a novel role of CCAR1 in DNA repair.
Cells constantly experience DNA damage throughout their lifespan. Particularly, double-stranded breaks (DSBs) can be detrimental if left unrepaired. Eukaryotes have developed mechanisms to detect and repair such damage, known as the DNA damage response (DDR). The DDR involves hundreds of key players and supporting factors. By utilizing a CRISPR screening system to assay about 18,000 gene knockdowns, we identified new players influencing homology-directed repair (HDR), a mechanism to repair DSBs. Among these, we focused on CCAR1, an enigmatic gene whose role in the DDR was not well understood. We found that reducing CCAR1 levels impaired both HDR and the repair of interstrand crosslinks (a type of DNA damage), similar to what occurs when the Fanconi anemia (FA) pathway is inactivated.
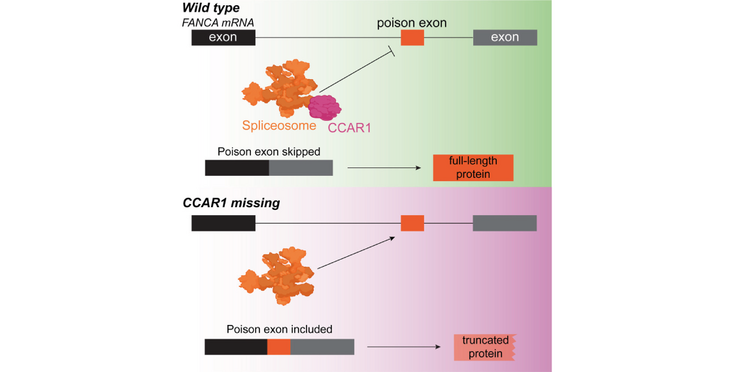
Interestingly, CCAR1 deficiency leads to FANCA (a critical FA pathway component) protein depletion without affecting FANCA mRNA or other FA gene mRNAs levels. Instead, CCAR1 prevents the inclusion of an exon with a stop codon (termed a poison exon) in the middle of the FANCA mRNA. This demonstrates that CCAR1 is crucial for proper mRNA splicing. Further analysis showed that CCAR1's role in mRNA splicing extends beyond FANCA, affecting many genes and ensuring that their mRNAs are correctly processed in both mouse and human cells. This highlights CCAR1's unexpected role in maintaining accurate mRNA splicing, which is essential for proper protein function.
Read the Paper in Molecular Cell (Open Access)
Figure from Karasu et al (2024) Mol Cell published under a CC BY 4.0 license.