The Roignant and Soller labs in collaboration with other groups including the Jinek lab found that the E3 ubiquitin ligase Hakai stabilizes the m6A methylation machinery. Their article entitled "Hakai is required for stabilization of core components of the m6A mRNA methylation machinery" has been published in Nature Communications.
Abstract
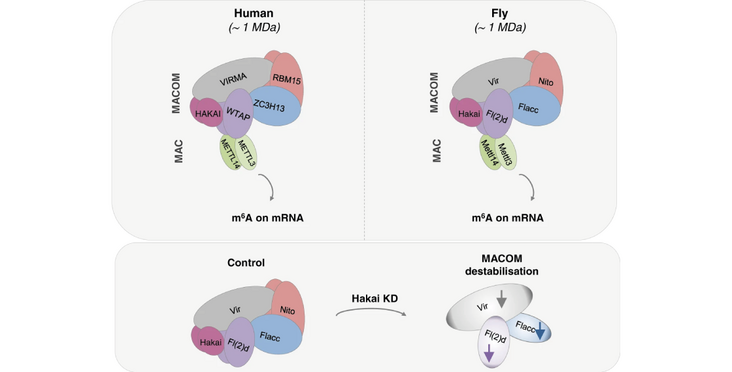
N6-methyladenosine (m6A) is the most abundant internal modification on mRNA which influences most steps of mRNA metabolism and is involved in several biological functions. The E3 ubiquitin ligase Hakai was previously found in complex with components of the m6A methylation machinery in plants and mammalian cells but its precise function remained to be investigated. Here we show that Hakai is a conserved component of the methyltransferase complex in Drosophila and human cells. In Drosophila, its depletion results in reduced m6A levels and altered m6A-dependent functions including sex determination. We show that its ubiquitination domain is required for dimerization and interaction with other members of the m6A machinery, while its catalytic activity is dispensable. Finally, we demonstrate that the loss of Hakai destabilizes several subunits of the methyltransferase complex, resulting in impaired m6A deposition. Our work adds functional and molecular insights into the mechanism of the m6A mRNA writer complex.
Read the Publication in Nature Communications
Abstract, figure and title from Bawankar, Lence et al. (2021) Nature Communications published under a CC BY 4.0 license.