A journey into non-canonical functions of Argonaute proteins
In eukaryotes, Argonaute (AGO) proteins are best known for their function in the miRNA and siRNA pathways. Besides this canonical function, Argonaute proteins were implicated in other cellular processes, primarily by experiments in cancer cell lines. Mouse embryonic stem cells (mESCs), derived from the inner cell mass of mouse blastocysts, are the only healthy cells surviving upon the depletion of mostmiRNA biogenesis factors. Working with this system, the Ciaudo lab (ETH Zurich), in collaboration with the Santoro lab (University of Zurich), could shed light on novel non-canonical functions of AGOs in early development.
In mESCs, only two of the four human AGOs, AGO1 & 2, are expressed. In these cells, the two AGOs have partially overlapping functions. In the absence of AGO2, AGO1 gets upregulated and loaded with miRNAs typically loaded into AGO2. But AGO1 cannot compensate for the lack of AGO2 in the extraembryonic endoderm differentiation. Hinting that AGOs have additional functions in early development besides their role in the miRNA pathway.
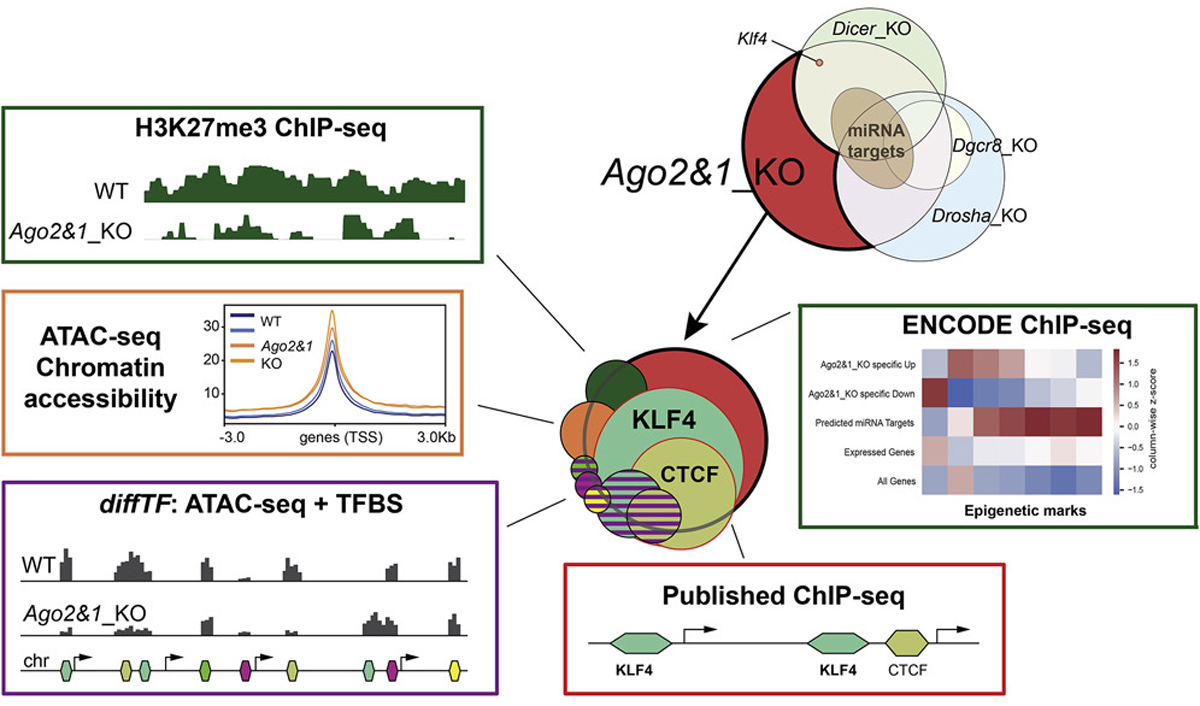
The journey started with the transcriptomic analysis of mESCs in which key players of the miRNA pathway were knocked out using CRISPR/Cas9 genome engineering approach. This analysis revealed that nearly 1800 genes show a changed expression only in AGO1 & 2 double-knockout cell lines but not a significant expression change in cells lacking either Dicer, Drosha or Dgcr8, indicating a miRNA-independent mode of regulation. Of these specifically differentially expressed genes (DEGs) in AGO1 & 2 double-knockout cells, over 1000 of them were downregulated and nearly 750 upregulated.
From this observation, the journey continued to elucidate how these DEGs are regulated. The first possibility investigated was the regulation by epigenetic modifications. The researchers assessed the global levels of specific histone marks. They found that overall levels of only one histone mark, H3K27me3, went drastically down in the absence of AGO1 & 2. Corresponding to this finding, several proteins forming the complex involved in its deposition showed reduced expression at the protein but not the RNA level. However, when looking at the genes most strongly regulated by this histone mark and comparing them to the AGO1 & 2 double-knockout specific DEGs, the overlap was small.
After ruling out histone marks as the primary regulators of these DEGs, the researchers looked at changes in chromatin accessibility in the AGO1 & 2 double-knockout cell lines. Over 3000 genomic regions showed changed accessibility only in the double AGO knockout and not in the single knockouts. However, the overlap of these regions with the genomic loci of the DEGs was again small.
Next, the researchers investigated the potential role of transcription factors (TFs) and their binding sites in the context of changed chromatin accessibility. This led to the identification of five TFs with computationally predicted differential binding in the double knockout cells. Then, they looked at the correlation of the differential TF binding sites with the genomic DEGs regions and found a positive correlation. Most of this correlation was contributed by the binding sites of the two TFs, KLF4 and CTCF.
While CTCF did not display changed at protein levels in the AGO1 & 2 double-knockout cells, KLF4 levels were significantly downregulated. Analyzing KLF4-ChIP-seq data, the researchers noticed that the promoter and enhancer regions of over 70 % of upregulated DEGs and nearly 50% of the downregulated DEGs were bound by KLF4. Intriguingly, this transcription factor is an important regulator of cellular pluripotency, which was now shown to be regulated by AGO1 & 2 in a miRNA-independent fashion. The researchers’ findings were published in the journal Stem Cell Reports.
“It was a journey through different fields: transcriptomics, epigenetics, genome organization and transcription factors, and integrating all the data took a lot of computational biology. The project’s success crucially dependent on the two co-first authors bringing in their respective expertise,” says Constance Ciaudo, last and corresponding author of the study. Regarding the continuation of the journey, Madlen Müller, co-first author comments: “I would like to find out the mechanism how AGOs control KLF4 expression and function. Also, which direct functions do Argonautes have in the nucleus and which effects observed there are indirect, is an intriguing open question.”
Publication:
Müller, Schäfer et al. (2022) Stem Cell Reports 17(5), 1070-1080 (Open Access)
Graphical abstract from Müller, Schäfer et al. (2022) Stem Cell Reports published under CC BY-NC-ND 4.0 licence.
Text: Dominik Theler