“There is no room for a zoo of modifications on mRNA.”

Chuan He gives us his perspective on the field of messenger RNA methylation and tells us about his career.
The field of mRNA methylation has publication wise exploded since 2011/2012 after what, in the case of m6A, seemed to be decades of relative dormancy. Why was this?
When m6A was discovered back in 1974, people recognized its importance because you had something so abundant on mRNA and the methyltransferase was found to be essential. I think that molecu- lar biologists in the 70ies and 80ies lacked the tools such as current mass spectrometry techniques for quantification of the chemical modification and importantly, sequencing technologies to map the modification sites and look at their distribu- tion. Back then cloning a gene was a PhD thesis and because there were no better approaches available, the field became sort of dormant. Still, few people were researching it mostly in plants and yeast, and I would say that the mainstream RNA community did not forget about it but somehow put it on the backburner. In 2008 my colleague Tao Pan and I started to think that there were well-known DNA and histone modifications playing critical roles in gene expression and, at the same time, RNA chemical modifications were as diverse if not even more diverse than pro- tein modifications but more than the ones on DNA. It was hard to believe that they mostly serve to fine-tune the structure and function and we thought that there had to be some additional roles. That led us to write a grant to study potential reversible RNA methylation, which was funded in 2009. My lab made a breakthrough in 2011 when we discovered that m6A is reversible and from there people started to jump in with modern technologies to map it and study the involved enzymes.
The currently known effects of m6A are diverse in affecting transcript stability, translational efficiency, and splicing. Do you have a sort of overreaching theory what the role of m6A is for the cell?
Regarding post-transcriptional regulation, I always argue that, on one hand translation initiation controls the entire proteome and, on the other hand, you have miRNAs regulating few or tens of genes. These two processes are critical but in mammalian systems, for example, during differentiation, development, signaling and stress response cells often change the expression of hundreds or even thousands of genes but you do not change the entire proteome, and you do not change only a few. So there seems to be a lack of a mechanism to coordinate this. Moreover, through animal experiments, it was discovered that if you get rid of this methylation machinery, most of the cells are stuck at the stem cell or progeni- tor stage. If you start looking into biolog- ical processes in animal models instead of HeLa and HEK cells, you realize the critical role m6A plays in cell differentiation. It has many roles, but the essential one is for cell differentiation and development. Mamma- lian systems are estimated to have around two hundred different cell types. Each cell type is thought to have its transcription program produced by waves of expression of various transcription factors that dictate lineage-specific cell differentiation and tissue maturation. Two hundred different cell types require thousands of such transcription factor waves regulating each up to hundreds and thousands of transcripts. We know how these several hundred transcripts are activated by transcription factors under each wave, but we do not how their translation and importantly their decay is coordinated. How could this be done? Conceptually the easiest way to think about this is to put a methyl group to coordinate several hundred transcripts under one wave providing them with a new identity and grouping them for coordinated decay before tackling the next wave. This was probably the initial role. Once you have something available, biology tends to take advantage of it and through evolution, it starts to pick up other functions. Fundamentally, I believe it is a way to coordinate the transcriptome for translation and decay.
What are currently the major open questions?
I think we currently know like thirty percent of what is going on mechanistically. There are still a lot of readers we have no knowledge on and we do not know about a lot of things occurring in the nucleus and cytoplasm. In the nucleus, we do not understand how specific transcripts are selected for methylation and the majority of the demethylation occur there. DNA methylation occurs at tens of millions of sites, but its demethylation concerns a few percents of these. RNA is the same thing. Concerning the demethylases, we do not know why they are in the nucleus and what they are doing. Importantly, if you knock out the methylase transferase and some of these demethylases in the nucleus, the phenotype is much more dramatic than when you knock out the cytoplasmic reader proteins acting in translation and turnover. There is something else fundamentally important going on in the nucleus that we do not quite understand. That is number one. Number two is the question, which I get all the time, where does the selectivity come from? How does the cell know this transcript needs to be methylated and importantly, how do the readers know these are the transcripts? How do these transcripts know they go to translation versus decay? These are questions not only for RNA methylation but also in general for RNA protein complexes. How do they know what set of RNAs experience what kind of environment in the cytoplasm which then leads to what kind of fate? I would say that this is still mostly a mystery.
“The mainstream RNA community did not forget about it but somehow put it on the backburner.”
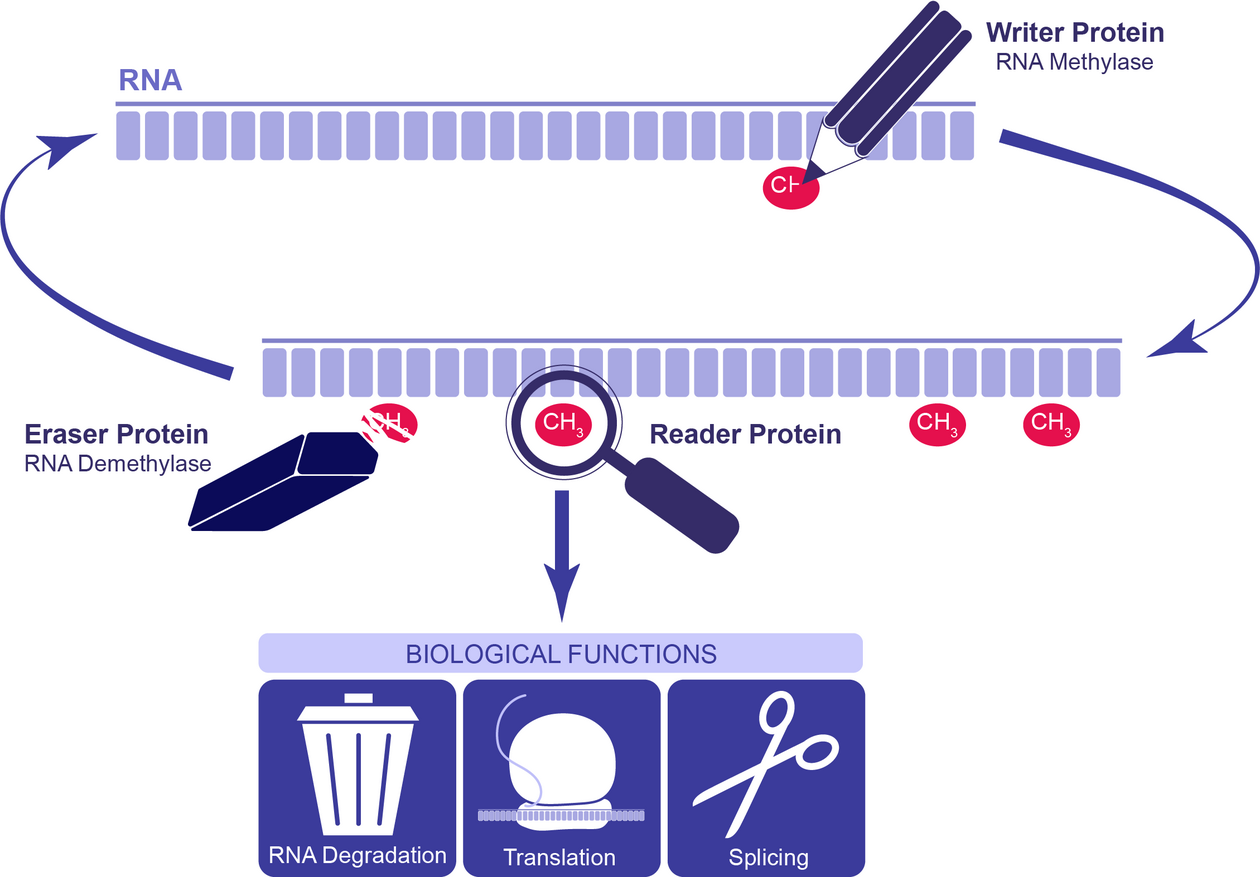
After the discovery that it is reversible, the term RNA epigenetics was coined: can it be called epigenetics in the sense that it has an effect on subsequent generations?
In 2010 I wrote a commentary in Nature Chemical Biology which was probably the first paper ever to propose the idea of dynamic reversible RNA methylation and the editor back then put RNA epigenetics into the title or text. I was a little hesitant back then, but if you look nowadays, it has a lot of common features with DNA methylation: there are writers, readers, and erasers. Not all methylation is erased but dynamically only to a portion, and it affects gene expression. What you do not have is the inheritance; it cannot be inheritable on the RNA level. Later on, people developed the term epitranscriptome, which I like very much, but back then, we were not as smart to come up with this. However, we do have unpublished results in the lab showing that RNA methylation could be “inheritable” through other mechanisms. Experiments in a model organism currently performed in my lab show that if we change RNA methylation, this leads to a dramatic phenotype not previously seen. When we remove the introduced perturbation, the phenotype stays. There is clearly something going on that we do not understand yet. In that sense in a few years maybe we can come back and revisit whether these phenomena are inheritable.
It is quite intriguing how similar the methyl binding pockets of the m6A reader domain YTH and the binding pockets of some histone methylation reader domains are. One could argue that they recognize chemically similar entities but could there also be domains reading out both?
I would be extremely cautious when people look at simple cell lines and declare that we understand everything. The thing I am most excited about right now in the lab is precisely what you just said. I even wrote a commentary for the journal of RNA in which I stressed the importance of what is going on in the nucleus. Before we thought, that transcription controls RNA methylation by methyltransferase recruitment, but they are coupled together. How could they be coupled together? One way would be through what you said multifunctional proteins, but it could also be through other pathways. Several hypotheses are currently tested in the lab. We have incredibly fascinating yet very preliminary results suggesting that RNA methylation could also affect chromatin state. Whether there are proteins capable of recognizing both, I do not know, but I can say that methyltransferases, as well as some of the nuclear readers, are impacting histone marks or other proteins that are related to altering chromatin structure.
Your lab published on other mRNA modifi- cations such as m1A and 2'-O methylation. Will it be discovered that there is a whole sort of zoo of RNA modifications as on rRNA and tRNA?
Well, one needs to be a little careful, there is no room for a zoo of modifications on mRNA, since it carries the genetic information from DNA to proteins, so you do not want to introduce modifications taking the chance of generating problems with translation. The other mRNA modifications are less abundant than m6A. For instance, m1A occurs in tissue at about five to ten percent of the level of m6A. Recently, two papers came out with single base resolution: there is a Nature paper showing about 20 sites, which is very interesting and nice work but it does not correlate with the mass spectrometry measurements of m1A’s abundance. There is another work in Molecular Cell, which maps a lot more sites in the 5’ UTR consistent with our study. Some of these abundant m1A sites are installed by tRNA methyltransferases, which were highjacked through evolution to act on mRNA. In our lab, we can confirm about six to seven different modifications on mRNA, and there could be other ones, which we do not know about yet. These other known ones are about ten percent as abundant as m6A and we suspect that many of them are installed by rRNA and tRNA modification en- zymes. For some, there are also seem to be dedicated modification enzymes.
“We can research this in HeLa and HEK cells to death, but if there is no biological relevance, there is no meaning to this.”
Is there more to come then?
Yes, but the essential one is m6A. For the rest, we have yet to see their functional impact on biology. It is important to study RNA methylation but the biological relevance is most crucial. We can research this in HeLa and HEK cells to death, but if there is no biological relevance, there is no meaning to this. At the end of the day, for these other modifications, we need to know what are their writers, readers and what the biological consequences in animals are when functional alterations occur.
“It cannot be inheritable on the RNA level.”
Is the m6A machinery a potential drug target or is it too essential?
It is druggable. Regarding m6A, some of the demethylases and reader proteins are not essential and with the right dosage, even the essential methyl transferase might be a drug target just like EZH and other histone modifiers. We start realizing that RNA methylation plays critical roles in cell differentiation but is also important for proliferation and growth. Recent results suggest that aberrant RNA methylation is another trick cancers use to gain a survival advantage. Therefore, the involved proteins can become potential targets for therapy. Industry started to jump into this, and several start-up companies are either formed or in the process of being created.
How was the transition from chemistry to a more life science focus for you and your lab?
I was trained as a synthetic chemist during my PhD at MIT, and after graduating, I was considering materials versus catalysis versus biology; and I saw this large potential in biology. I then did a couple of years of biochemistry during my postdoc, and when I started my lab, we were doing mostly synthetic work because that was what I was most comfortable with, but we always performed biological experiments on the side. We were working a little bit on DNA damage, structural biology and then switched to five years of microbiology, which also included genetic work. In 2008 I got tenure, which is a milestone in one’s career, I closed down my chemistry lab because I did not feel like I can make the type of contribution that would satisfy me in the chemistry field. For the first eight years of my career from chemistry to structural biology and microbiology, I was continually looking for something that could potentially give me an identity and allow me to leave a mark in science. Then epigenetics came, which from a chemistry point of view makes sense: In the human genome, you have three billion base pairs, we have tens and trillions of cells and the complexity has to come from chemical modifications. The diversity and complexity of chemical modifications of biological macromolecules were the general direction we decided to go into. Tao Pan and I used to have lunch every week talking about this and inspiring each other and so we decided to explore RNA modifications and from there on, we were very lucky with the findings we made starting with the reversibility of m6A methylation.
What made you initially become a scientist?
In China, for kids of my generation doing well in school it was almost a straightforward path to become scientists. In college, I started realizing that I wanted to do something which is quite flexible, challenging and intellectually rewarding. Only later in my career, I began appreciating the type of freedom you have as a scientist to explore your interests. I also dislike when things are foreseeable and in science, you do not know where you stand in a couple of years.
Who had a significant influence on your career?
My generation was inspired by Chinese-American Nobel prize recipients. They were like national pride. Then my parents, who are not scientists but well educated, provided me with a good environment. Back then, there were not so many options for young people in China as today. It concerns me that, also in the US, many talented young kids s do not go into science anymore.
What caused this change?
The business world, which makes it easier for people to be awarded for their work and compensated better. It has become less trendy to go into science, and young people tend to go more into business, IT, finance and consulting. Business is taking over the world in a way. I do not blame the young people, but at the same time, science also needs young talents.
Do you encourage your kids to go into science?
I do, but my wife has different thoughts when she looks at the lives of scientists, which are quite stressful. I do not know about researchers in Switzerland, but in the US, it is stressful and very competitive. Five to ten percent of the time you are enjoying the rewards, but most of the time you work hard, it is stressful, and you travel a lot.
How do you perceive the increasing competition to acquire funding in the USA?
I feel bad when I started as an assistant professor the funding was fine with an approval rate of around twenty percent, and there were plenty of private foundations supporting young researchers. I think that the biomedical research has gone a little bit out of control in the US. The NIH budget has doubled and kept increasing. That was great for biomedical research, but at the same time, every medical school and university involved tried expanding it. University faculty members have stable support as they teach, but there are many soft money places doing great science, but the expansion has gone a little bit too fast. The result is that at most biomedical research institutes everybody complains about the lack of funding. Yet everybody is guilty of trying to expand it. I wished the system had undergone more steady progress instead of this rapid expansion. It does dramatically drive life science forward, but concomitantly creates so many PhD students and postdocs. Nowadays it is unfortunate that it takes six to eight years in some biological disciplines to get a PhD and another five to six years as a postdoc before becoming an assistant professor. By that time, you are close to forty years old and even older when you obtain your first NIH R01 grant, which is a very competitive process. That turns back many young people when they look at this. I do not think that this is necessary and I wish there were ways to shorten the time to obtain a PhD, and the postdoc does not need to be so long. Of course, we want NIH to keep increasing funding, but at the same time, I would wish that research institutions and universities would think twice before they expand. Many places realized the problem now and tied up their budgets and expansion plans.
Biography of Chuan He

In 2000, Chuan He obtained his PhD in chemistry from the Massachusetts Insti tute of Technology, Cambridge under the supervision of Stephen J. Lippard. He then moved to the lab of Gregory L. Verdine at Harvard University for a postdoc and in 2002 became an assistant professor at the University of Chicago. In 2008, he was promoted to associate professor and in 2010 to full professor. He is the director of the Institute for Biophysical Dynamics at the University of Chicago and the Synthetic and Functional Biomolecules Center at Peking University. In 2014, he became an investigator of the Howard Hughes Medical Institute.
Interview: Dominik Theler