Protein polymerization preventing pathological aggregation
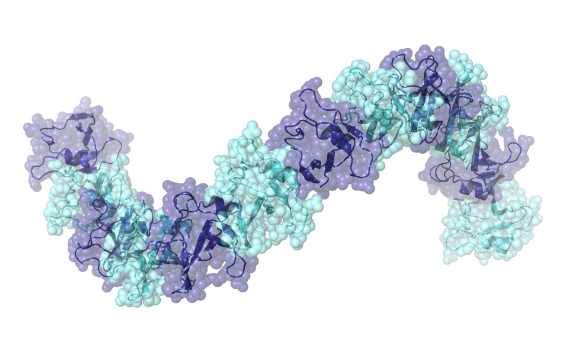
There are still a lot of unanswered questions when it comes to the pathogenic effects of proteins in connection with ALS and dementia. Tariq Afroz from the Polymenidou group at the University of Zurich has now found a novel and unique higher order structure of RNA-binding proteins: the oligomerization of TDP-43, mediated by its N-terminal domain.
TDP-43 is one of the key factors when it comes to understanding the molecular mechanism of Amyotrophic lateral sclerosis (ALS) as well as a special form of dementia (Frontotemporal dementia, FTD). The protein has been shown to bind both DNA and RNA and has many functions in transcriptional repression, pre-mRNA splicing and translational regulation. A previous study by Polymenidou and colleagues revealed that thousands of RNAs are bound by TDP-43 in neurons.
A lot of work has been invested to unravel the molecular mechanisms behind the pathogenic effects of TDP-43. The protein is usually present in the cell nucleus only, but in patients with ALS and FTD it leaks out of the cell nucleus, and aggregates in the cytoplasm, thus catalysing a damaging chain of events inside the cell and causing it to die. A group around Magdalini Polymenidou from the Institute of Molecular Life Sciences, University of Zurich, and Frédéric H.T. Allain from the Institute of Molecular Biology and Biophysics of the ETH Zurich has now reported that physiological nuclear TDP-43 in mouse and human brain forms homo-oligomers that are resistant to cellular stress. The oligomerization is mediated by the N-terminal domain (NTD) of TDP-43, which can adopt dynamic, solenoid-like structures. This was revealed by a 2.1Å crystal structure in combination with nuclear magnetic resonance spectroscopy and electron microscopy. The group provides evidence that the physiological state of the protein in the nucleus is in fact oligomeric and that this oligomerization is indispensable for the functional role of the protein in RNA metabolism.
Initially the NTD of TDP-43 was thought to be unstructured. Intriguingly, while several recent studies highlighted the importance of NTD for functional TDP-43 dimerization and nucleic acid interaction, others argued that the same domain promoted pathologic aggregation and neurotoxicity. Moreover, a small fraction of TDP-43 was reported to exist as dimers in cells, so the dimers were suspected to initiate or “seed” the formation of pathologic TDP-43 aggregates.
TDP-43 oligomerization and its mediation by NTD is novel and unique among the higher order structures described for RNA-binding proteins. This leads to a range of attractive hypotheses: First, TDP-43 binds RNA preferentially as an oligomer to increase affinity towards long contiguous UG-repeats. Moreover, TDP-43 oligomerization may act as a “recruitment platform” for speci?c factors involved in splicing and RNA maturation steps important for sustaining their levels. And there is another potential role of inter-molecular TDP-43 interaction that is NTD-driven: The protein has the ability to bring distal sites on RNA in close proximity, resulting in looping-out of RNA. Depending on the binding sites, this mechanism may promote inclusion or skipping of alternative exons.
One important thing remains enigmatic, though: The mechanism of transition of physiological nuclear TDP-43 to pathologic, irreversible and insoluble protein assemblies, leading to neurodegeneration. It is known that TDP-43 contains a C-terminal prion-like or low-complexity domain (LCD), mediating protein–protein interactions, and that this LCD is crucially involved in disease. So the group suggests that the transition is likely triggered by the proteolytic release of its C-terminal LCD, which harbours most of the ALS-linked mutations and indeed possesses high propensity to self-associate, phase separate, and aggregate. The collected data strongly supports the hypothesis that formation of physiological NTD-mediated, nuclear TDP-43 oligomers can counteract cytoplasmic aggregation. Any factor altering the cellular equilibrium between oligomeric and monomeric TDP-43 may trigger the initiation of cytoplasmic TDP-43 aggregation.
Afroz T. et al. (2017) Nature Communications 8, 45 (open access)
By Roland Fischer