Interview with Thomas R. Cech

“For them, it was a weird organism doing a weird thing”
In the interview, Thomas R. Cech discusses his groundbreaking discovery regarding the catalytic properties of RNA, the RNA world hypothesis, recent developments in the field of RNA biology, his career and current research topics as well as the situation of the American research community after Trump’s election.
Dr. Cech, you launched a revolution in RNA Biology by discovering the catalytic properties of self-splicing ribosomal RNA, since then the field has exploded …
... not only because of my work. I was near the beginning of that revolution; other people were working on RNA splicing, which is a fundamental process still being researched today. The splicing community thought that this was pretty cool what we were doing and supported us.
You knocked over the dogma that RNA is only the carrier of information. Can you remember an anecdote?
The RNA people were very excited in a positive way. They had been speculating much earlier about catalytic properties of RNA. In the context of the origin of life, it was an interesting hypothesis for genetic material to evolve, if it could replicate itself. This group included some of the world leaders such as Francis Crick and Leslie Orgel. Now that we had shown that RNA had catalytic activity, this fitted nicely with those people saying RNA should be able to do everything. It was just an idea, but now we had real solid evidence. The broader community was more skeptical and did not care so much, because we found this just in this little pond animal called Tetrahymena. So, it did not shake their tree too much at the time. For them, it was a weird organism doing a weird thing. But when there were many more examples found, then, of course, some people got more concerned about it.
There is a whole zoo of non-coding RNAs. Do we know what they are doing in the cell?
Absolutely not, it will take another century. In human cells, perhaps several hundred thousands of these non-coding RNAs exist, which compares to only 20’000 messenger RNA genes. Therefore, there are a lot more different non-coding RNAs than messenger RNAs. The first question to ask is: How many of these are even functional? Some of them could be just transcriptional noise in the cell, the improper making of RNA or transcribed junk DNA that remains without function. Yes, for some of them that could be true. For other ones, it may just be the act of making the RNA in a specific region of the chromosome that helps other genes in that neighborhood to stay active; so the particular RNA itself is not important, but rather the act of making it there. Then there are those cases where the RNA product of these junk regions is not junk but it is doing something exciting, important, and maybe even medically relevant for human health. Therefore, those are the ones that people are trying to discover and focus on. We are in very early times of researching this, so it is best to be open-minded.
“ That RNA was at the origin of life is hard to prove, because it is a historical question rather than a scientific question.”
Has the RNA world hypothesis now been proven?
No, that RNA was at the origin of life is hard to prove, because it is a historical question rather than a scientific question. Even if a scientist could show that you could make life from RNA today in the test tube, this would still not prove that this is how it happened.
It is hard to think of a way how you would prove it since in contrast to dinosaurs and trilobites, these RNA molecules do not leave fossil evidence. We can come up with reasonable stories, which make sense, but that does not prove that they are correct.
How did the new gene editing technologies change the field of RNA biology?
CRISPR is very easily used in human cells grown in culture and can as well be used to make mice that have precise changes in their chromosomes. This is a godsend for the RNA community because we can prevent one of these non-coding RNA from being made quite quickly and see whether that has any consequences. In many cases, researchers have found that the RNA does have a function since you see a phenotype if you knock out the RNA. Often protein coding genes stop working if you prevent the neighboring non-coding RNA from being produced. Some of these protein-coding genes are medically really important since they are involved in heart development and disease. The majority of these non-coding RNAs are involved in enhancer and promoter regions, while others work at a greater distance, and so for those, it may not be so obvious to elucidate their mechanism of action
Can you elaborate on the role of RNA in epigenetics?
This is a fascinating topic in the context of gene silencing. At the level of proteins which condense and de-condense the chromatin, most of these epigenetic complexes bind RNA in living systems. They bind many RNAs, and this RNA binding promotes or represses their activity. Some of these complexes bind RNA promiscuously. That is what we have recently found, which is one kind of story, where the RNA binding prevents the chromatin-modifying complex from turning off a gene. Therefore, the RNA acts as an inhibitor of an inhibitor. If the RNA is being made in a particular part of the chromosome, then you want to keep this chromatin silencing complex away; so, if that is the mode of action, do you want the RNA to be very specific? No, then it would only work in one place. So you have to be general, and the chromatin modifying complex binds just any RNA it encounters. The RNA then removes it from the chromosome so that it no longer can silence that particular gene. Once there is no RNA present the complex can come and silence the gene.
What new surprises can we expect in the field of RNA biology in the next few years?
For the last 30 years, we have underestimated every time this question came up, what surprises would still come along. People thought, oh now, we are leveling off, most of the discoveries have been made, and now things will go more slowly. Instead then, for example, CRISPR or long intergenic non-coding RNAs (lincRNAs) come along, and all of a sudden open questions in the field are once again exploding. Some of the questions are: How important are lincRNAs for the brain? Are these maybe contributing to the cognitive power of primates and humans? Therefore, one can imagine, that such a thing could explode in the future; but we do not know.
What are you working on right now?
My lab mainly works on telomerase and an epigenetic silencing complex. Joachim Lingner, who is now a professor at EPF Lausanne , discovered that telomerase is a reverse transcriptase, which was the first example of such an enzymatic activity in eukaryotic cells. This discovery caused an explosion in that field with currently 1000 publications published every year about telomerase, which is a medically very important protein, but about this, we had no idea at the time of that discovery.
How does telomerase work?
It builds out the ends of our chromosomes. In the absence of telomerase, the chromosome ends get a little bit shorter with each cell division. Once these ends get too short, this sends a signal to the cell to quit dividing; this is a good thing for most of the cells in our body. If one did not have this process, we would be enormous now, because all of the cells in our body would continue dividing. However, cancer cells find a way to reactivate the telomerase enzyme so that they can keep dividing and this is not a good thing for us. On the other hand, there are the stem cells in our body, which are essential for tissue regeneration and have to continue dividing at a modest rate. Therefore, they need to have telomerase activity, and there are quite some human diseases occurring when stem cells do not have enough telomerase activity. This leads to problems with the body’s blood supply, lungs, skin, and early aging symptoms. This is an uncomfortable situation, where we have a telomerase activity as a critical biological component: Having too much of it is like having one foot in the door for cancer, having too little of it, you get into early aging. Thus, it has to be kept under accurate control, and that is why you go one direction or the other when the control sometimes goes bad. We are trying to understand how that works.
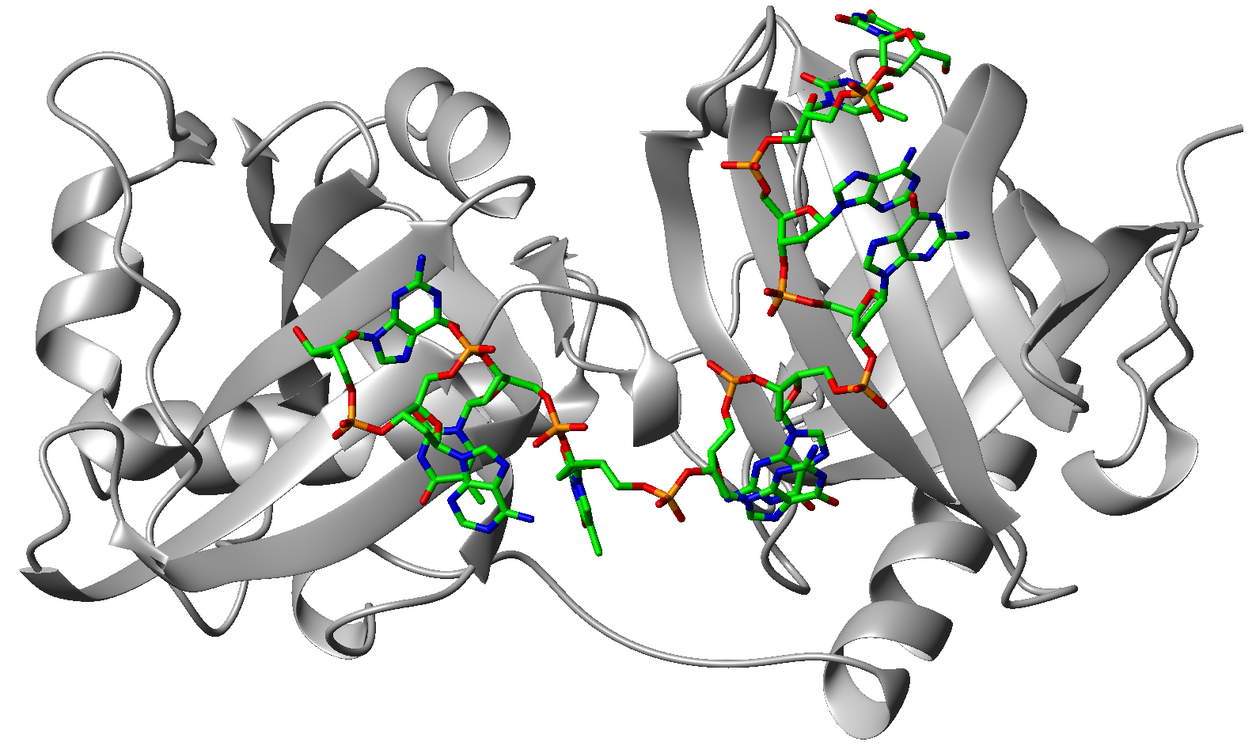
protein bound to a telomere derived DNA sequence (PDB entry: 1XJV),
Lei M. et al. (2004) Nature Structural & Molecular Biology 11, 1223–1229.
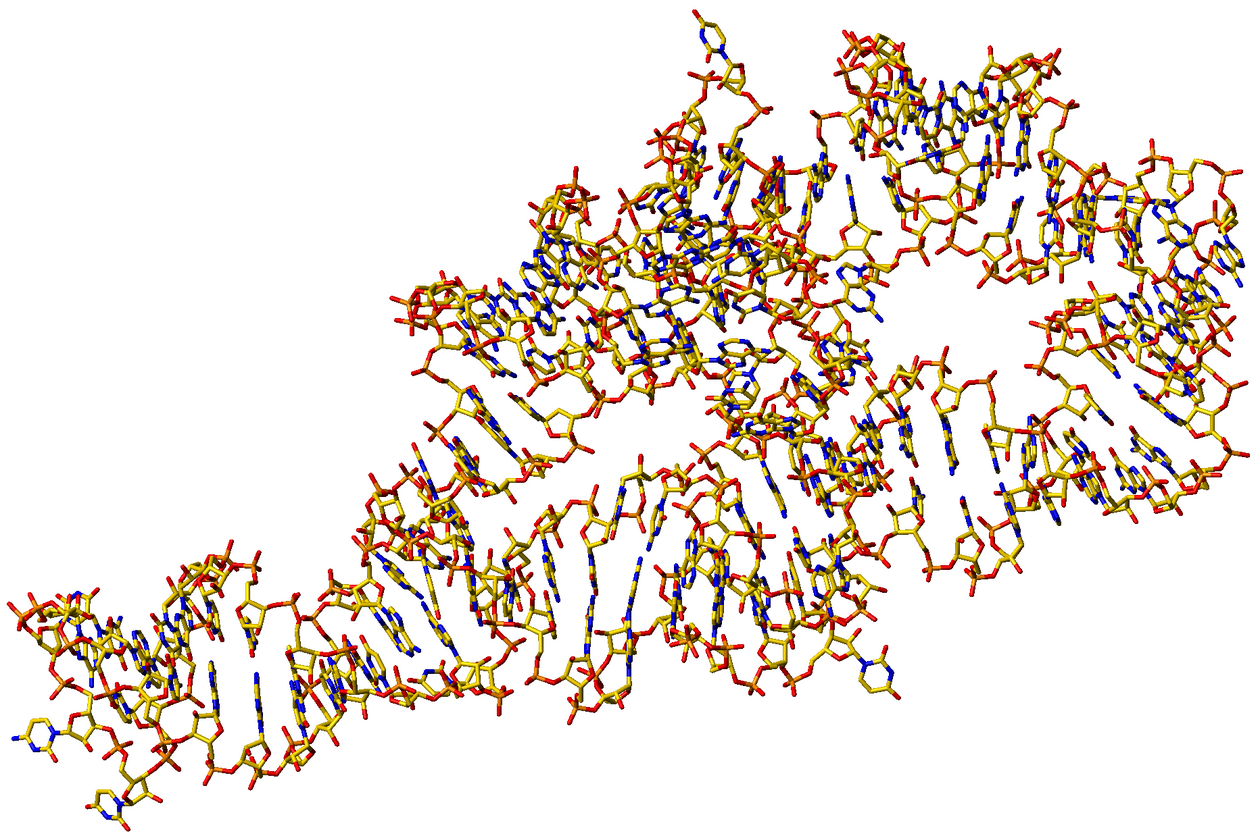
Cate J.H. et al. (1996) Science 273 (5282).
Can this knowledge be used to develop new cancer drugs?
There are many companies and academic laboratories working on telomerase inhibitors; that could be a useful anti-cancer approach. In the clinics currently, it is more being used diagnostically, since you sometimes have mutations in the reverse transcriptase subunit that activate the telomerase. Patients that have these mutations in the regulatory promoter region do very badly regarding cancer progression. Medical doctors are using this diagnostic information regarding telomerase mutations to decide whether to apply a harsher chemotherapy or not. Telomerase mutations are not the only marker for aggressiveness of a given cancer, but it is helpful. In 70% of all melanoma samples, you detect these mutations.
“ We are in very early times of researching non-coding RNAs, so it is best to be open-minded.”
RNA medicine is a buzzword, where does it stand now?
For a long time, it was just a good idea. However, there were not many examples of success. Just last year the FDA (Food and Drug Administration) approved a new treatment for SMA (Spinal Muscular Atrophy), which is an antisense RNA drug directed against an RNA process, which was developed by Ionis together with Biogen. It worked well enough that they had to stop the clinical trial early because they saw so much success with the kids that were getting the drug. This treatment is life-saving for these children, but we do not yet know whether it will allow them to live healthy lives, but it is very promising. To me as a non-expert, the drug seems to be quite specific and therefore relatively safe. It is a costly treatment.
There are 150 ongoing clinical trials with RNA therapeutics. Which ones are the most promising?
The CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology requires RNA, and these are probably right now the most talked about RNA therapeutics. The development of the antisense approach being usable as a drug took a long time. RNA itself is not that great of a drug, because it is a large molecule with many negative charges on it, making delivery into cells challenging and by itself is not stable at all. Those problems have been overcome better and better over the years through chemical modifications introduced into the RNA.
Your former lab member Jennifer Doudna was one of the leading scientists in developing the CRISPR technology for genome engineering.
Jennifer Doudna was a postdoctoral fellow in the mid-90s at the same time as Joachim Lingner was in the lab. This was an exciting and fruitful time when she determined the 3-D-structure of a non-coding RNA. We knew that this RNA could act like an enzyme and therefore imagined that it must have a particular shape, but we only had a vague idea of what it might look like. She was able to determine its structure at the atomic level. It was fascinating for us to see the architecture of this RNA. Once this happened, there was a revolution in RNA structural biology, including the ribosome and the spliceosome structures. This pushed people! Jennifer Doudna was very famous already before CRISPR: She got elected to the US National Academy of Sciences at an extremely young age and promoted to full professor at Yale in record time, which she left to become a full professor at Berkeley. She was a winner from the beginning. It is now maybe not even so unexpected that she made another big discovery. I do not know if I can keep up with her (laughs).
You were president of the HHMI, why did you step down after nine years?
I enjoy teaching and love training students how to become scientists. At the HHMI (Howard Hughes Medical Institute) I was an administrator of a huge organization and would mostly talk to lawyers, finance and investment people. I was quite removed from the action of doing science and teaching. After a decade, I felt it was time to step down even though I loved it.
Today, you are still heading a lab at age 69?
You think I should retire (laughs)? I know that in Switzerland, I would be retired for four years. There is no perfect system; in the US system, there can be people that are very old, not at the height of their talent anymore, who still stick around and absorb money that younger people could use.
“ I knew I am going to be a scientist from second grade.”
How did you get hooked on science?
I knew I am going to be a scientist from second grade, because my parents saved everything I ever wrote. I wrote about being a scientist at that age. The reason was genetic in my case, probably inherited from my father who was a medical doctor, but always wished he could be a physicist. In fact, his hero was Albert Einstein, and he would have loved coming to Berne to see the Einstein house.
In College, you first chose to study the humanities. Why did you then switch to chemistry?
It is wonderful as a young person to have a broad education, because it sets you up for your life, to be a good citizen, a good parent. When it comes to making a living, I could not see doing this by reading the great books. So for a profession, I always knew, it would be science. Currently, I am reading a new translation of Dante’s Inferno. I am about halfway through, and things are getting bad going down towards hell.
What was your reaction when Trump got elected?
That was very surprising to many of us! A similar surprise as in the UK with the Brexit vote. Many of us had underestimated how much dissatisfaction and even anger there was in large parts of the population. They were ready for a major change. I must say though; I come to Europe quite often, there is an undercurrent of similar thinking in many European countries. Many people that are anti-immigration, who are suspicious of people with a different background, who are thinking the economy is not helping them. I think these things could happen in Europe as well as many of the manufacturing jobs have gone to Asia. The second idea I had: maybe some of Trump’s ideas are pretty good such as reducing the size of the government.
Wait, wait! Trump is so much anti-science, anti-vaccine, anti-climate change.
Oh yes, this is very worrisome. The vaccine part is particularly disturbing: because of all of the medical advances in the 20th century, it is the single one that made the most difference regarding lives saved. Antibiotics would rank very high, but only second to vaccines. In our parent’s generation, polio was such a devastating disease. Kids would go to school in the morning, have a fever at night and might be dead or paralyzed the next day. People have forgotten how quickly the vaccines turned this around. The irony is that those vaccines were not safe by today’s standards. Vaccines have an extremely high bar for being approved because you are going to give them to a large number of small children who are healthy. Therefore, if there is any chance that they are harmful, they will not be approved. Despite the very rigorous approval process, these people just make up this stuff. In fact, the original worry turned out to be fraudulent, but on the internet, this stuff persists. There are always some parents whose kids get autism, and since we do not understand the cause of autism, they say: maybe it is the vaccine. So maybe it is one of a hundred other things, too; it is very disturbing.
Will there be a brain drain in the US?
I think it is possible that many of our students who come from Asia or the Middle East are feeling unwelcome because they are subjected to so much negative scrutiny. So I am afraid that we may lose many of our talented graduate students as well. However, I am an optimistic person and think, we can turn this around, but of course, I am concerned about it at this time.
Do you fear cuts in the National Institute of Health’s budget?
There is a proposal from the president’s budget that it will be severely decreased. Initially, 25%, now the proposal is back to 5%. It turns out 5% is huge, since much of the money is already committed, so it has a much larger effect on the current year’s budget. For young people who want to get started, you have to multiply by about five; we are talking a 25% decrease in a particular year that would be devastating. HHMI and the Chan-Zuckerberg Initiative (Facebook) are doing a fantastic job to find novel ways to support creative science in the biomedical area. That makes a difference, but it is hard for any private philanthropy to cover the NIH budget, which is much larger.
For the deconstruction of American science, the president can do only so much without the support of the Congress, which has to appropriate the money. There is a great deal of support for medical research in the Congress. I think the area that I am in is unlikely to be decimated, but I do worry about climate research. Two thousand scientists work in Boulder in government labs studying the climate such as NOAA (National Oceanic and Atmospheric Administration), NCAR (National Center for Atmospheric Research), and the NREL (National Renewable Energy Lab). In these areas, you can shut down with an executive order from the president certain kinds of research without the Congress being involved - I should not say this because I do not want to give anyone any idea. George W. Bush did this with stem cell research in 2001, prohibiting federal funding of research. It is a worrisome time for them; they feel like a Damocles sword hanging over them and are quite concerned.
Biography Prof. Thomas R. Cech

After obtaining his Ph.D. in chemistry from the University of California, Berkeley and postdoctoral research at the Massachusetts Institute of Technology, Dr. Cech joined the faculty of the University of Colorado Boulder in 1978. In 1982 Dr. Cech and his research group discovered self-splicing RNA in Tetrahymena, providing the first exception to the long-held belief that biological reactions are always catalyzed by proteins. Because RNA can be both an information-carrying molecule and a catalyst, perhaps a primordial self-reproducing system consisted of RNA alone. For the discovery of RNA’s catalytic properties, he was awarded the 1989 Chemistry Nobel Prize together with Sidney Altman. From 2000 – 2009 Dr. Cech served as the president of the Howard Hughes Medical Institute, which is the largest private biomedical research organization in the USA. He is a member of the National Academy of Sciences and directs the BioFrontiers Institute at the University of Colorado Boulder.
This interview was conducted by Nik Walter (Head science section Tages-Anzeiger ) in the context of Thomas Cech’s visit to Switzerland as a speaker in the NCCR RNA & Disease seminar series. Read the portrait of Thomas Cech written by Nik Walter entitled “Der Dogma-Brecher” on the Tages-Anzeiger or Bund Website. The portrait pictures were kindly provided by Adrian Moser (Chief photographer Der Bund). Depictions of the structures were generated with the program MOLMOL (Koradi R. et al. (1996) Journal of Molecular Graphics, 14(1), 51-55.