If it’s complex, it’s possibly quadruplex
‘If G-quadruplexes form so readily in vitro, Nature will have found a way of using them in vivo’, said Nobel prize winner Aaron Klug already some decades ago. The Hall group from the Institute of Pharmaceutical Sciences at ETH Zurich has now clarified one of these functions: the control of the polyamine biosynthesis pathway by G2-quadruplexes.
G-quadruplexes are naturally-occurring structures found in RNAs and DNAs. Over the past two decades biologists and bioinformaticians have unearthed substantial evidence for G-quadruplexes as important mediators of biological processes. This includes telomere damage signaling, transcriptional activity, and splicing. Although their structures are difficult to characterize in vivo, G-quadruplexes are recognized as important elements regulating gene expression, and they are increasingly linked to diseases. As in DNA, regular RNA G-quadruplexes have shown to be highly stable due to stacked planar arrangements connected by short loops. More interestingly still, reports of irregular qua- druplex structures are increasing and recent genome-wide studies suggest that they influence gene expression. Thousands of such motifs have been identified, the majority of which comprised canonical short-looped G3-tracts (G3-quadruplexes). Stable G3-qua- druplexes (i.e. strong enough to stall reverse transcriptase) in eukaryotic cells have been shown to be frequently unwound; so the physiological relevance of quadruplex structures should not be automatically inferred from their stability. Consistent with this, several hundred putative metastable RNA G2-quadruplexes have also been predicted throughout the transcriptome. So far, few G2-quadruplexes have been studied in detail biophysically, structurally and functionally.
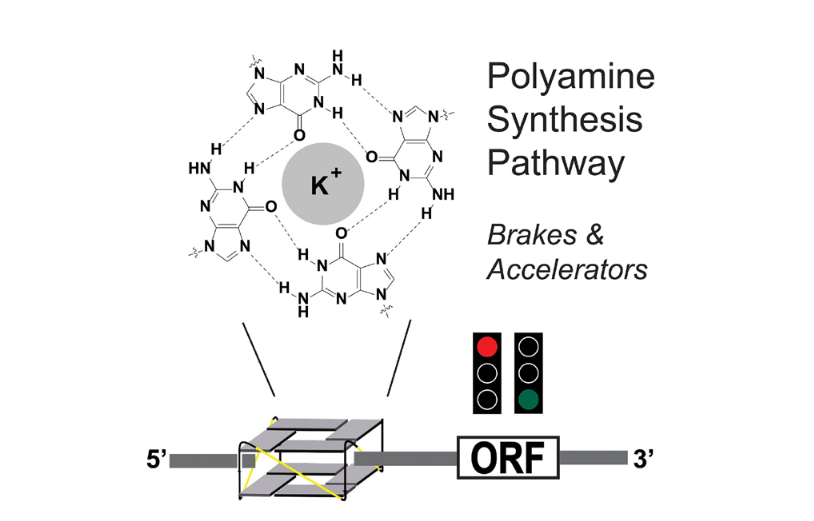
As reported in the open-access journal eLife, the Hall group has investigated a grouping of G2-motifs in the untranslated regions (UTRs) of eight genes involved in polyamine biosynthesis, and concluded that several likely form novel metastable RNA G-quadruplexes. They performed biophysical characterizations of their properties, comparing them to a reference G-quadruplex and discovered how some of these motifs are able to regulate and sense polyamine levels, creating feedback loops during polyamine biosynthesis. With the key help from the Allain group, the team demonstrated using NMR spectroscopy that one particular long-looped quadruplex in the AZIN1 mRNA co-exists in salt-dependent equilibria with a hairpin structure.
The group identified 35 putative G2-tract quadruplex structures in the 5’ and 3’ UTRs of genes in the polyamine (PA) biosynthesis pathway. Using cellular reporter assays they could identify twelve of these covering eight PA synthesis proteins that altered reporter activity in comparison to mutants. Strikingly, most of these structures had the effect to reduce PA levels. This suggested they might act in unison as regulatory elements to control PA homoeostasis.
The study thus expands the repertoire of regulatory G-quadruplexes and demonstrates how they act in unison to control metabolite homeostasis. More specifically, the group’s findings reveal a previously unrecognized mechanism of PA self-regulation. They expect that such mechanisms through G-quadruplexes may be a common feature in other metabolic pathways.
Lightfoot et al., (2018) eLife 7:e36362.
By Roland Fischer