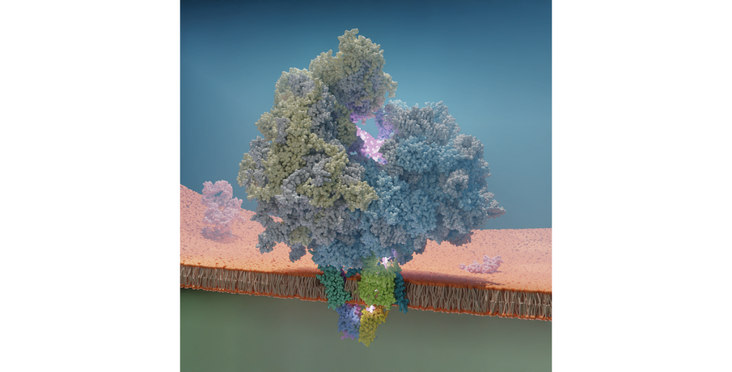
A recent "Nature Structural & Molecular Biology" paper by the Ban (IMBB) and Deuerling (University of Konstanz, Germany) groups unveils the molecular architecture of the TRAP complex in the endoplasmic reticulum, revealing its participation in protein biogenesis and hormone secretion.
The translocon-associated protein (TRAP) complex plays a critical role in protein biogenesis and hormone secretion, such as insulin.A study by Jaskolowski et al. uncovers the molecular architecture of the TRAP complex, which spans the endoplasmic reticulum (ER) membrane, and its interaction with Sec translocons and translating ribosomes. Cryo-EM analysis and in vivo experiments in C. elegans led researchers to propose a model for how TRAP is involved in ER protein biogenesis. They discover previously uncharacterized TRAPa anchor interactions with the ribosome, which help target ribosomes to the ER. When the ribosome binds the translocon, TRAP fully engages the ribosome, positioning its hydrophobic cradle below the translocon pore exit for interactions with emerging nascent polypeptides.
The hydrophobic features of the TRAP cradle with potential chaperoning function, are found to be crucial for the biogenesis of secreted and membrane proteins in the ER. The TRAPa subunit is identified as the key subunit of the complex with a dual role in providing an anchor for ribosome attachment and a hydrophobic cradle-like domain to facilitate protein folding and biogenesis.
TRAP complex likely works alongside other protein complexes in the membrane and might occasionally reposition itself to grant access to other nascent chain interacting factors. This study reveals how TRAP could achieve that through its flexible anchor attachment, enhancing our understanding of TRAP's role in protein biogenesis and translocation in the ER.
Read the Publication in Nature Structural & Molecular Biology (Open Access)