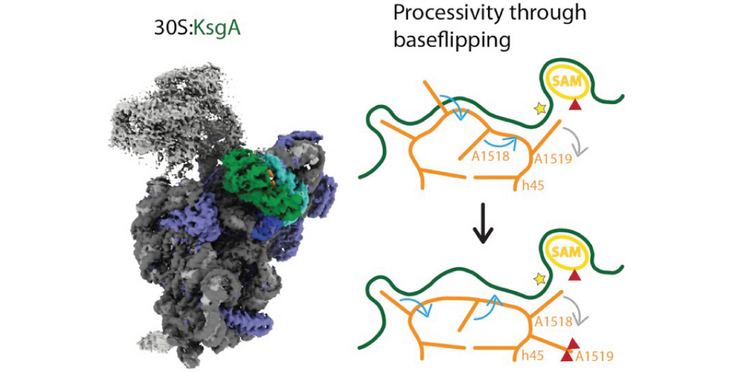
The Ban lab (D-BIOL, ETH Zurich) determined the structure of the E. Coli 30S ribosome in complex with the conserved methytransferase KsgA, of which Dim1 is the eukaryotic homologue. Their structure allowed the researchers to suggest that the enzyme subsequently modifies two adjacent sites without dissociating from the ribosomal subunit. Their findings have been published in an Nucleic Acids Research article entitled "Structural basis of successive adenosine modifications by the conserved ribosomal methyltransferase KsgA".
Abstract
Biogenesis of ribosomal subunits involves enzymatic modifications of rRNA that fine-tune functionally important regions. The universally conserved prokaryotic dimethyltransferase KsgA sequentially modifies two universally conserved adenosine residues in helix 45 of the small ribosomal subunit rRNA, which is in proximity of the decoding site. Here we present the cryo-EM structure of Escherichia coli KsgA bound to an E. coli 30S at a resolution of 3.1 Å. The high-resolution structure reveals how KsgA recognizes immature rRNA and binds helix 45 in a conformation where one of the substrate nucleotides is flipped-out into the active site. We suggest that successive processing of two adjacent nucleotides involves base-flipping of the rRNA, which allows modification of the second substrate nucleotide without dissociation of the enzyme. Since KsgA is homologous to the essential eukaryotic methyltransferase Dim1 involved in 40S maturation, these results have also implications for understanding eukaryotic ribosome maturation.
Read the Publication in Nucleic Acids Research (Open Access)
Abstact, figure and title from Stephan et al. (2021) Nucleic Acids Research published under a CC BY-NC 4.0 license.