A paper with co-corresponding authors Franka Voigt (Chao lab, FMI) and Tomás Aragón (University of Navarra) provides new insights into non-canonical splicing of XBP1 by IRE1a required for the Unfolded Protein Response (UPR).Tthrough using single molecule live cell imagining approaches, the researchers confirmed that unspliced XBP1 mRNA associates stably with the ER and this targeting relies on translation taking place. The researchers also found that the splicing of the XBP1 mRNA leads to its removal from the ER membrane and its transcripts do not co-localize which large IRE1a clusters which were observed only upon overexpression of IRE1a. Their findings have been published in the eLife article "Live imaging of the co-translational recruitment of XBP1 mRNA to the ER and its processing by diffuse, non-polarized IRE1a".
Abstract
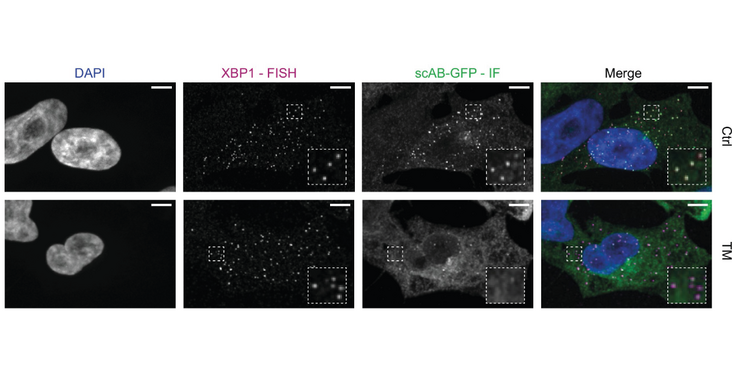
Endoplasmic reticulum (ER) to nucleus homeostatic signaling, known as the unfolded protein response (UPR), relies on the non-canonical splicing of XBP1 mRNA. The molecular switch that initiates splicing is the oligomerization of the ER stress sensor and UPR endonuclease IRE1a (inositol-requiring enzyme 1 alpha). While IRE1acan form large clusters that have been proposed to function as XBP1 processing centers on the ER, the actual oligomeric state of active IRE1a complexes as well as the targeting mechanism that recruits XBP1 to IRE1a oligomers remains unknown. Here, we have developed a single-molecule imaging approach to monitor the recruitment of individual XBP1 transcripts to the ER surface. Using this methodology, we confirmed that stable ER association of unspliced XBP1 mRNA is established through HR2 (hydrophobic region 2)-dependent targeting and relies on active translation. In addition, we show that IRE1a-catalyzed splicing mobilizes XBP1 mRNA from the ER membrane in response to ER stress. Surprisingly, we find that XBP1 transcripts are not recruited into large IRE1a clusters, which are only observed upon overexpression of fluorescently tagged IRE1a during ER stress. Our findings support a model where ribosome-engaged, immobilized XBP1 mRNA is processed by small IRE1a assemblies that could be dynamically recruited for processing of mRNA transcripts on the ER.
Read the Publication in eLife (Open Acess)
Abstract, figure and title from Gómez- Puerta et al. (2022) eLife published under a CC BY 4.0 license.