A recent "Nature Chemical Biology" paper by the Allain group (IMBB) in collaboration with the Jeschke (DCHAB-PC) and Klotzsch (HEST & Humboldt) labs demonstrates a novel method to simultaneously quantify FUS in the droplets and characterize FUS structurally both in the dispersed and condensed phases.
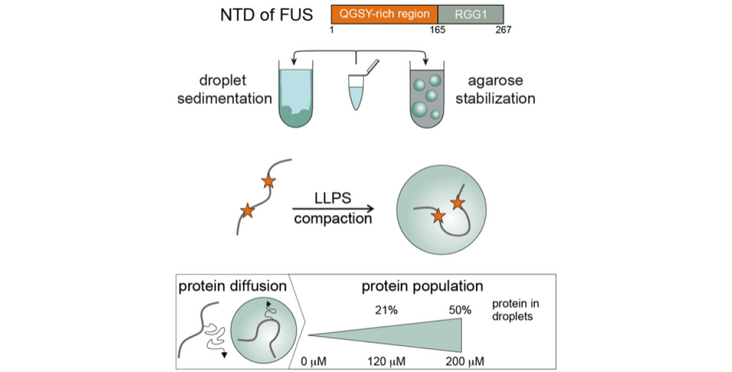
Many RNA-binding proteins undergo liquid-liquid phase separation, which underlies the formation of membraneless organelles, such as stress granules and P-bodies. Studies of the molecular mechanism of phase separation in vitro is hampered by coalescence and sedimentation of organelle-sized droplets that interact with glass surfaces. Here we demonstrate that liquid droplets of FUS, which is a protein found in cytoplasmic aggregates of ALS and FTD patients, can be stabilized in vitro using an agarose hydrogel that acts as cytoskeleton mimic. This allows their spectroscopic characterization by liquid phase NMR and electron paramagnetic resonance (EPR) spectroscopy. Protein signals from both dispersed and condensed phases can be observed simultaneously and their respective proportions be quantified precisely. Furthermore, the agarose hydrogel acts as a cryoprotectant during shock freezing which facilitates pulsed EPR measurements at cryogenic temperatures. Surprisingly, Double Electron-Electron Resonance (DEER) measurements revealed a compaction of FUS in the condensed phase.
Read the Publication in Nature Chemical Biology
Figure and text kindly provided by the autors.
Both were published as well as News on the website of ETH's D-BIOL.