The Chao lab's findings on the structure and function of the uS19 C-terminal tail during have just been published in Cell Reports in an article entitled "Dynamics of uS19 C-Terminal Tail during the Translation Elongation Cycle in Human Ribosomes".
Highlights
- uS19 C-terminal tail undergoes dynamic rearrangement during translation elongation
- uS19 C-terminal tail stabilizes the P-site tRNA and decoding interactions
- Disease-associated mutations in uS19 C-terminal tail cause translational defects
Summary
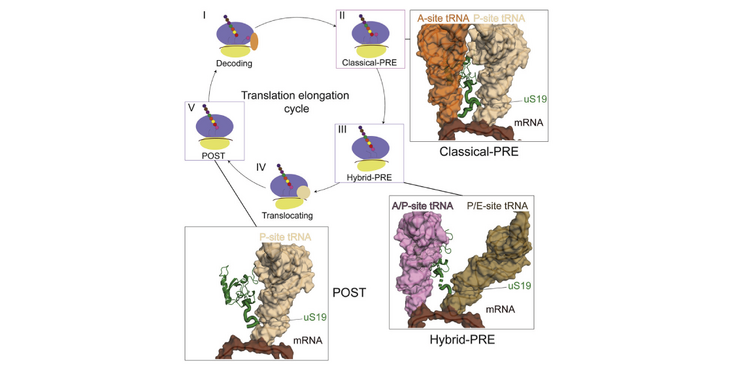
Ribosomes undergo multiple conformational transitions during translation elongation. Here, we report the high-resolution cryoelectron microscopy (cryo-EM) structure of the human 80S ribosome in the post-decoding pre-translocation state (classical-PRE) at 3.3-Å resolution along with the rotated (hybrid-PRE) and the post-translocation states (POST). The classical-PRE state ribosome structure reveals a previously unobserved interaction between the C-terminal region of the conserved ribosomal protein uS19 and the A- and P-site tRNAs and the mRNA in the decoding site. In addition to changes in the inter-subunit bridges, analysis of different ribosomal conformations reveals the dynamic nature of this domain and suggests a role in tRNA accommodation and translocation during elongation. Furthermore, we show that disease-associated mutations in uS19 result in increased frameshifting. Together, this structure-function analysis provides mechanistic insights into the role of the uS19 C-terminal tail in the context of mammalian ribosomes.
Read the Publication in Cell Reports (Open Access)
Graphical abstract, highlights and summary from Bhaskar V. et al. (2021) Cell Reports published under a CC BY-NC-ND 4.0 license.