The Chao lab investigated by single-molecule translation site imagining the role that the proteins LARP1 and 4EBP1/2 play in translational repression in general of mRNAs and of mRNAs containing a 5'TOP motif whose translation is regulated by mTORC1. They found that 4EBP1/2 has plays a dominant role in repressing the translation of all types of mRNAs, while LARP1, in contrast to a recent proposal, does not selectively control the translation of 5'TOP mRNAs but rather protects them from degradation. Their findings were published in the article "Distinct roles of LARP1 and 4EBP1/2 in regulating translation and stability of 5'TOP mRNAs" in Science Advances.
Abstract
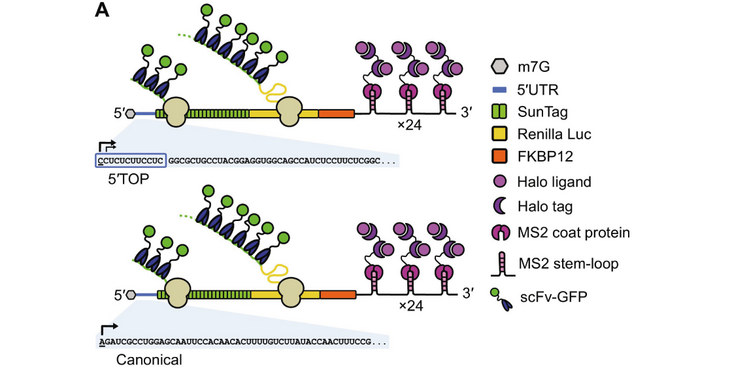
A central mechanism of mTOR complex 1 (mTORC1) signaling is the coordinated translation of ribosomal protein and translation factor mRNAs mediated by the 5'-terminal oligopyrimidine motif (5'TOP). Recently, La-related protein 1 (LARP1) was proposed to be the specific regulator of 5'TOP mRNA translation downstream of mTORC1, while eIF4E-binding proteins (4EBP1/2) were suggested to have a general role in translational repression of all transcripts. Here, we use single-molecule translation site imaging of 5'TOP and canonical mRNAs to study the translation of single mRNAs in living cells. Our data reveal that 4EBP1/2 has a dominant role in repression of translation of both 5'TOP and canonical mRNAs during pharmacological inhibition of mTOR. In contrast, we find that LARP1 selectively protects 5'TOP mRNAs from degradation in a transcriptome-wide analysis of mRNA half-lives. Our results clarify the roles of 4EBP1/2 and LARP1 in regulating 5'TOP mRNAs and provide a framework to further study how these factors control cell growth during development and disease.
Read the Publication in Science Advances (Open Access)
Abstract, figure and title from Hochstoeger et al (2024) Sci Adv published under a CC BY-NC 4.0 license.