The group of NCCR junior principal investigator Magdalini Polymenidou (Institute of Molecular Life Sciences, University of Zurich) published their findings on the different localization of FUS in neurons and astrocytes under hypertonic stress in a Cell Reports paper. The group of former NCCR junior principal investigator Marc-David Ruepp (King’s College London) contributed to the study.
Highlights
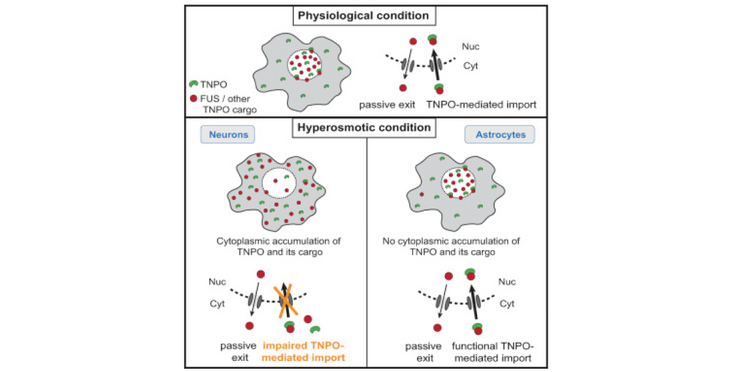
- Hypertonic stress drives cytoplasmic relocation of endogenous FUS in neurons
- FUS shifts to the cytoplasm independently of stress granules or hyperosmolar pathways
- FUS translocation results from insufficient nuclear import by transportin
- Astrocytes are spared from stress-induced FUS translocation and pathology in FTD-FUS
Summary
The primarily nuclear RNA-binding protein FUS (fused in sarcoma) forms pathological cytoplasmic inclusions in a subset of early-onset amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) patients. In response to cellular stress, FUS is recruited to cytoplasmic stress granules, which are hypothesized to act as precursors of pathological inclusions. We monitored the stress-induced nucleocytoplasmic shuttling of endogenous FUS in an ex vivo mouse CNS model and human neural networks. We found that hyperosmolar, but not oxidative, stress induced robust cytoplasmic translocation of neuronal FUS, with transient nuclear clearance and loss of function. Surprisingly, this reaction is independent of stress granule formation and the molecular pathways activated by hyperosmolarity. Instead, it represents a mechanism mediated by cytoplasmic redistribution of Transportin 1/2 and is potentiated by transcriptional inhibition. Importantly, astrocytes, which remain unaffected in ALS/FTD-FUS, are spared from this stress reaction that may signify the initial event in the development of FUS pathology.
Read the Publication in Cell Reports (Open Access)
Graphical abstract, highlights and summary from Hock E.-M. et al. (2018) Cell Reports, 24(4), 987-1000 published under the CC BY 4.0 license.