Using Cryo-EM, the Ban lab could visualize eight assembly intermediates of the human mitochondrial large ribosomal subunit and determined molecular structures for three of these. Their findings were published in Nature Communications in an article with the title "Stepwise maturation of the peptidyl transferase region of human mitoribosomes ".
Abstract
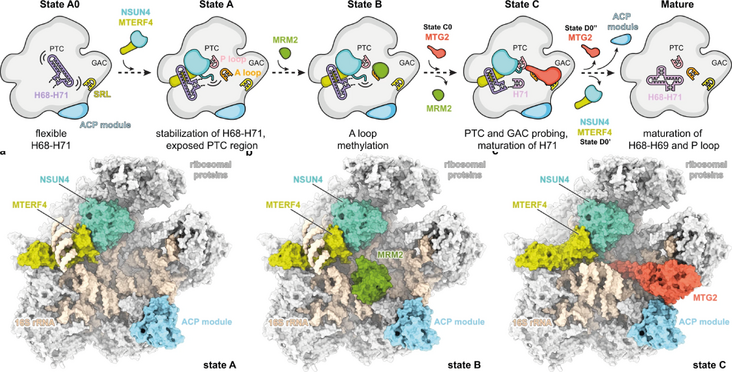
Mitochondrial ribosomes are specialized for the synthesis of membrane proteins responsible for oxidative phosphorylation. Mammalian mitoribosomes have diverged considerably from the ancestral bacterial ribosomes and feature dramatically reduced ribosomal RNAs. The structural basis of the mammalian mitochondrial ribosome assembly is currently not well understood. Here we present eight distinct assembly intermediates of the human large mitoribosomal subunit involving seven assembly factors. We discover that the NSUN4-MTERF4 dimer plays a critical role in the process by stabilizing the 16S rRNA in a conformation that exposes the functionally important regions of rRNA for modification by the MRM2 methyltransferase and quality control interactions with the conserved mitochondrial GTPase MTG2 that contacts the sarcin-ricin loop and the immature active site. The successive action of these factors leads to the formation of the peptidyl transferase active site of the mitoribosome and the folding of the surrounding rRNA regions responsible for interactions with tRNAs and the small ribosomal subunit.
Read the Publication in Nature Communications (Open Access)
Abstract, figures and title from Lenarcic, Jaskolowiski et al. (2021) Nature Communications published under a CC BY 4.0 license.