The NCCR RNA & Disease member labs of Nenad Ban, Oliver Mühlemann and Volker Thiel joined forces to study how SARS-CoV-2 alters translation. The preprint entitled "SARS-CoV-2 Nsp1 binds ribosomal mRNA channel to inhibit translation " describing their findings has just been posted on bioRxiv.
Abstract
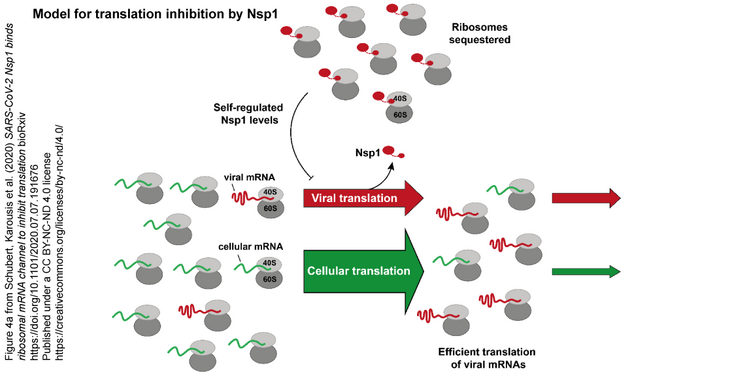
The non-structural protein 1 (Nsp1), also referred to as the host shutoff factor, is the first viral protein that is synthesized in SARS-CoV-2 infected human cells to suppress host innate immune functions. By combining cryo-electron microscopy and biochemical experiments, we show that SARS-CoV-2 Nsp1 binds to the human 40S subunit in ribosomal complexes including the 43S pre-initiation complex. The protein inserts its C-terminal domain at the entrance to the mRNA channel where it interferes with mRNA binding. We observe potent translation inhibition in the presence of Nsp1 in lysates from human cells. Based on the high-resolution structure of the 40S-Nsp1 complex, we identify residues of Nsp1 crucial for mediating translation inhibition. We further show that the full-length 5' untranslated region of the genomic viral mRNA stimulates translation in vitro, suggesting that SARS-CoV-2 combines inhibition of translation by Nsp1 with efficient translation of the viral mRNA to achieve expression of viral genes.
Download the Preprint from bioRXiv
Figure 4a and abstract from Schubert, Karousis et al. (2020) SARS-CoV-2 Nsp1 binds ribosomal mRNA channel to inhibit translation bioRxiv Published under a CC BY-NC-ND 4.0 license.