The Ban and Shan labs' insights into "Molecular mechanism of cargo recognition and handover by the mammalian signal recognition particle" and its regulation have been published in Cell Reports.
Highlights
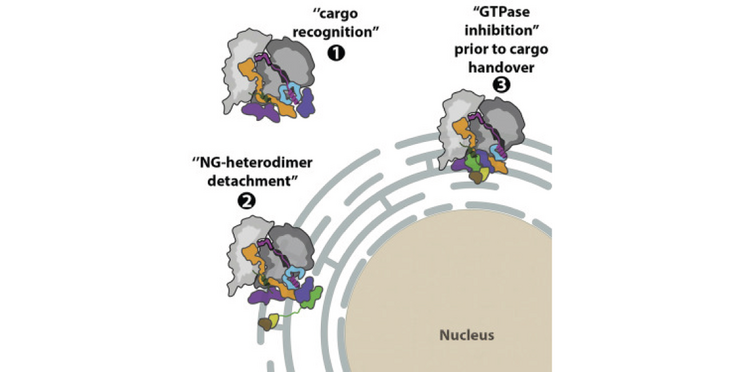
- Cryo-EM reveals the molecular features of the mammalian SRP targeting complexes
- The fold of the eukaryotic SRP54 M domain and signal sequence binding is revealed
- SRP·SR complex reveals eukaryotic-specific SRP contacts at the distal site
- Regulation of the SRP·SR GTPase occurs through conserved protein and RNA contacts
Summary
Co-translational protein targeting to membranes by the signal recognition particle (SRP) is a universally conserved pathway from bacteria to humans. In mammals, SRP and its receptor (SR) have many additional RNA features and protein components compared to the bacterial system, which were recently shown to play regulatory roles. Due to its complexity, the mammalian SRP targeting process is mechanistically not well understood. In particular, it is not clear how SRP recognizes translating ribosomes with exposed signal sequences and how the GTPase activity of SRP and SR is regulated. Here, we present electron cryo-microscopy structures of SRP and SRP·SR in complex with the translating ribosome. The structures reveal the specific molecular interactions between SRP and the emerging signal sequence and the elements that regulate GTPase activity of SRP·SR. Our results suggest the molecular mechanism of how eukaryote-specific elements regulate the early and late stages of SRP-dependent protein targeting.
Read the Publication in Cell Reports (Open Access)
Graphical abstract, highlights, summary and title from Jomaa et al. (2021) Cell Reports published under a CC BY-NC-ND 4.0 license.