The Polacek lab discovered that a specific rRNA expansion segment of the yeast ribosome recruits three tRNA synthetases. However, deletion of this segment did neither significantly alter ribosome biogenesis & translation nor the activity of these tRNA synthetases raising the possiblity that they perfom additional functions when associated with ribosomes beside their core function. Their publication "tRNA Synthetases Are Recruited to Yeast Ribosomes by rRNA Expansion Segment 7L but Do Not Require Association for Functionality " has been published in the non-coding RNA Journal.
Abstract
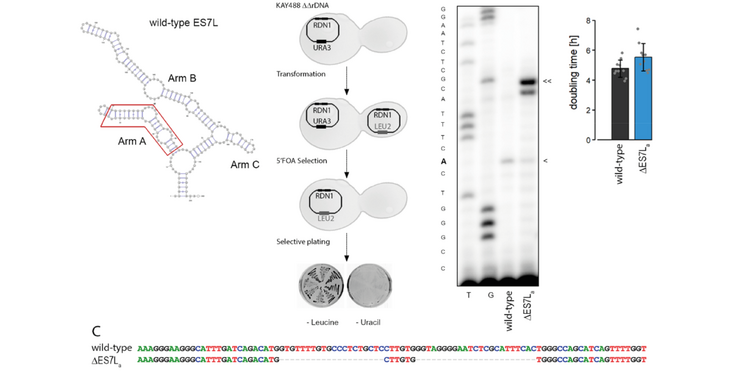
Protein biosynthesis is essential for any organism, yet how this process is regulated is not fully understood at the molecular level. During evolution, ribosomal RNA expanded in specific regions, referred to as rRNA expansion segments (ES). First functional roles of these expansions have only recently been discovered. Here we address the role of ES7La located in the large ribosomal subunit for factor recruitment to the yeast ribosome and the potential consequences for translation. Truncation of ES7La has only minor effects on ribosome biogenesis, translation efficiency and cell doubling. Using yeast rRNA deletion strains coupled with ribosome-specific mass spectrometry we analyzed the interactome of ribosomes lacking ES7La. Three aminoacyl-tRNA synthetases showed reduced ribosome association. Synthetase activities however remained unaltered suggesting that the pool of aminoacylated tRNAs is unaffected by the ES deletion. These results demonstrated that aminoacylation activities of tRNA synthetases per se do not rely on ribosome association. These findings suggest a role of ribosome-associated aminoacyl-tRNA synthetase beyond their core enzymatic functions.
Read the Publication in Non-Coding RNA (Open Access)
Abstract, figure and title from Krauer et al. (2021) Non-Coding RNA published under a CC BY 4.0 license.