The Stoffel lab (D-BIOL, ETHZ) investigated the role of miR-802 in the homeostasis of the mouse small intestine. Their findings have been published in a Nature Communications article entitled: "miR-802 regulates Paneth cell function and enterocyte differentiation in the mouse small intestine".
Abstract
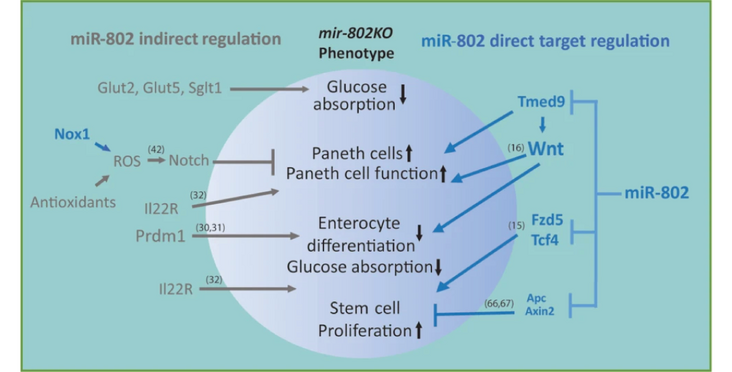
The intestinal epithelium is a complex structure that integrates digestive, immunological, neuroendocrine, and regenerative functions. Epithelial homeostasis is maintained by a coordinated cross-talk of different epithelial cell types. Loss of integrity of the intestinal epithelium plays a key role in inflammatory diseases and gastrointestinal infection. Here we show that the intestine-enriched miR-802 is a central regulator of intestinal epithelial cell proliferation, Paneth cell function, and enterocyte differentiation. Genetic ablation of mir-802 in the small intestine of mice leads to decreased glucose uptake, impaired enterocyte differentiation, increased Paneth cell function and intestinal epithelial proliferation. These effects are mediated in part through derepression of the miR-802 target Tmed9, a modulator of Wnt and lysozyme/defensin secretion in Paneth cells, and the downstream Wnt signaling components Fzd5 and Tcf4. Mutant Tmed9 mice harboring mutations in miR-802 binding sites partially recapitulate the augmented Paneth cell function of mice lacking miR-802. Our study demonstrates a broad miR-802 network that is important for the integration of signaling pathways of different cell types controlling epithelial homeostasis in the small intestine.
Read the Publication in Nature Communications (Open Access)
Abstract, figure and title from Goga et al. (2021) Nature Communications published under a CC BY 4.0 license.